The fight against Streptococcus suis has been a long-time struggle for swine producers, veterinarians, and researchers worldwide. S. suis is an important disease responsible for meningitis, septicemia and other invasive pathologies mainly in post-weaned piglets. S. suis varies in its genetic makeup around the world complicating its diagnostic and epidemiological surveillance. S. suis is also an important globally emerging zoonotic disease for swine industry workers (occupational disease). In some regions (most notably certain Asian countries), it is a frequent cause of serious disease outbreaks in humans exposed to diseased animals or contaminated pork products. Currently, the use of antibiotics is being limited all over the world – meaning swine producers are going to have to depend on prevention methods other than prophylactic/metaphylatic use of antibiotics. So why is there no effective commercial vaccine against Streptococcus suis? (Segura M., 2015).

All about Streptococcus suis
Streptococcus suis is naturally present in the upper respiratory tract of pigs, as well as the digestive and genital tracts. Up to 100% of pigs in a herd are carriers of this bacterium, meaning they are colonized without demonstrating clinical signs. That said, these carrier pigs can still pass on the bacteria to other animals (Gottschalk M, Segura M., 2019).
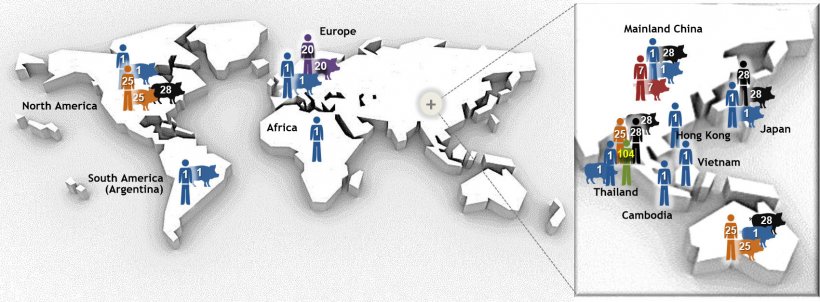
S. suis exists worldwide and differs greatly from region to region (Figure 1). The bacterium was originally classified into 35 serotypes defined by the sugars found in the “capsule” surrounding the bacterial surface (a few of these serotypes are now being debated as whether or not belonging to the S. suis species). Yet, the most common serotypes recovered form pig clinical cases are 2 (worldwide), 9 (certain European countries), 3, 1/2, and 7 (mainly North America; and Asia for serotype 3 as well). S. suis is also classified in “sequence types”, which are based on the bacterium ‘DNA fingerprint’ (Goyette Desjardins, et al. 2014). Each serotype of S. suis therefore contains numerous sequence types (Figure 1). All this diversity means that individual S. suis infections have unique characteristics in terms of serotype, sequence type, zoonotic potential and clinical outcomes. This huge amount of variation helps explain why it is so difficult to create one “universal” vaccine that protects against all S. suis infections in pigs worldwide (Goyette Desjardins, et al. 2014).
Types of vaccines
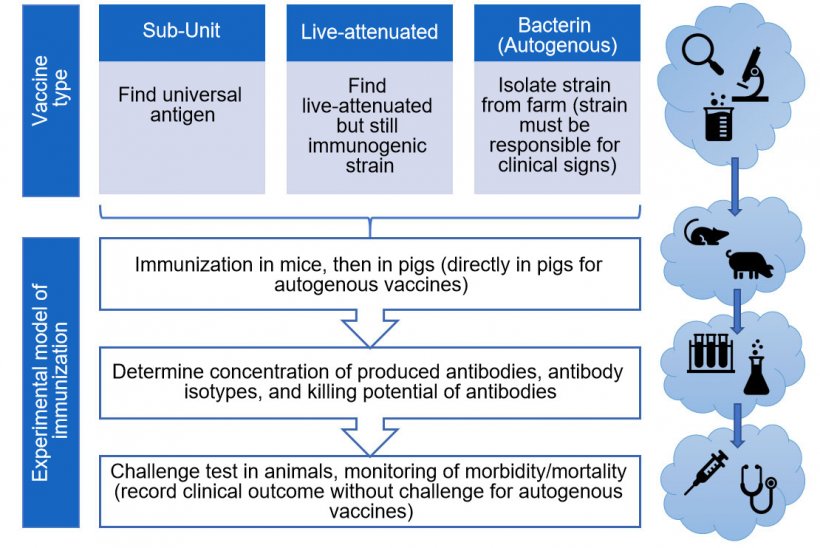
There are numerous types of vaccines, all with their own pros and cons. Animals can be protected by injecting them with either a component (sub-unit) of the bacterium, live-attenuated bacteria or killed (inactivated)-bacteria. Experimental S. suis sub-unit vaccines seem promising, but require strong adjuvants (solutions to amplify the immune system response). Additionally, because S. suis is so diverse, finding a specific component (Ex. a protein) able to protect against all S. suis strains remains a challenge. The combination of different S. suis proteins (antigens) in a sub-unit vaccine would likely afford the best chance of a more “universal” and effective protection. On the other hand, while live-attenuated vaccines offer the hypothetical benefits of requiring no boosters or adjuvants, they present a public health risk, given that S. suis is zoonotic and that the injected strain could revert to virulence. The second drawback of S. suis live-attenuated vaccines is that naturally or experimentally infected animals produce low levels of antibodies, so it is difficult to envisage that an attenuated strain (which can be easily eliminated by the host) will be able to induce a protective response when a virulent strain is not able to do so. Indeed, this fact might explain why a multiple-injection protocol was used in most live-attenuated vaccine studies (Segura M., 2015).
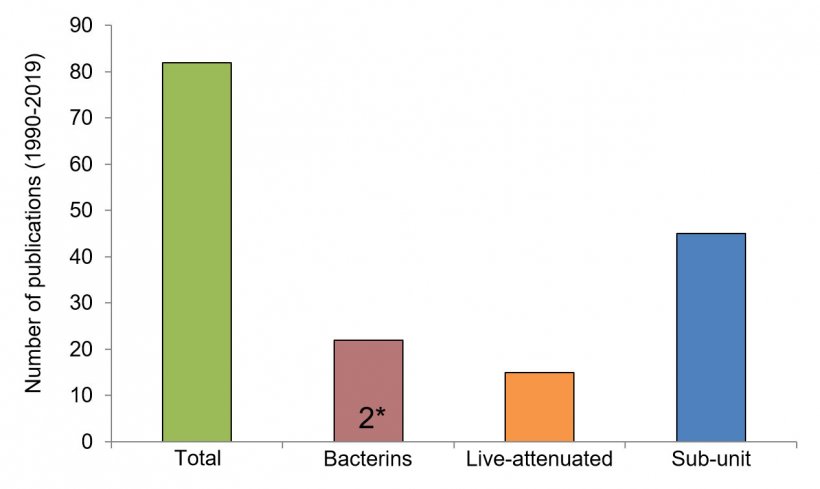
The last type of vaccines commonly evaluated for S. suis prevention are killed (inactivated)-bacteria or “bacterins”, limiting the public health risk – but also their ability to stimulate the immune system, and thus providing controversial results (Segura M., 2015). Actually, autogenous bacterins represent the only available option in the field. These vaccines are bacterins prepared for a specific farm by sampling animals from the affected herd. As such, despite the huge variation in S. suis infections by region, vaccinated animals are protected from the same strain(s) causing clinical problems within the herd in question. However, the diagnosis of S. suis as a primary cause of disease may complicate the choice of the strain(s) to be included in the autogenous vaccine. That said, more investigations of all types of vaccines are necessary before drawing any conclusions on which presents the ultimate solution. As of 2019, the vast majority of publications on S. suis immunization studied sub-unit vaccines, then bacterins, and finally live-attenuated vaccines (Figure 2).
Challenges in S. suis vaccine development
There is currently no international agreement on how vaccine efficacy should be tested, meaning that it is very difficult to compare the results of different formulations. Not only is there variation among studies with respect to the vaccine formulation, boosters, and immunized animals (sows vs. piglets) but also in what measures are taken to determine that a vaccine is protecting animals after all! Classifying the antibodies into their isotypes (or sub-class of antibodies) is important to allow a prediction of the type of immune response generated by immunization: the ideal response leads to bacterial destruction. In the case of S. suis, this effect can be measured by a “killing assay”; which ensures that vaccine-produced antibodies are functional. Functionality depends on the antibody isotype produced: not all of them are able to induce S. suis elimination. However, there is currently no standardized protocols for testing S. suis vaccine efficacy (e.g. animal model, challenge infection, killing assays, etc.), further contributing to the confusion surrounding interpretation of vaccine trial results (Segura M., 2015). For example, of the 17 studies on S. suis vaccines published between 2015 and 2019, the majority tested for the presence of antibodies and performed mortality assessments in mice. Not even half of the aforementioned studies performed killing assays (and of these, the methodology used varied greatly), and/or the analysis of the type(s) of antibodies produced. Even fewer performed a morbidity/mortality assessment or tests in pigs! Of note: while vaccination of mice offers interesting predictive power in the case of negative results, promising vaccine candidates must absolutely be tested in pigs under controlled experimental challenge conditions (Figure 3). Yet, experimental S. suis infection of conventional piglets under laboratory conditions gives inconsistent results, provoking another drawback in vaccine development. Indeed, most S. suis serotypes are unable to cause any clinical signs under experimental conditions. In the case of autogenous vaccines, reports are almost inexistent (only 2 published papers in the last 30 years, Figure 2) or incomplete and, in most of them, a control (non-vaccinated) group is missing in order to afford scientifically sound conclusions (Segura M., 2015).

Unfortunately, vaccine testing itself is not the only area where knowledge is lacking. More studies on maternal antibody interference are necessary in order to determine conclusively whether it is preferable to vaccinate sows or piglets, and when? It is key to find the optimal window for piglet vaccination; after antibodies passed on by the sow have disappeared, but before the piglet is totally unprotected (and thus vulnerable to infection). Finally, the chosen vaccine also needs to be practical for large-scale application; minimizing the number of necessary boosters, prioritizing immunization of sows rather than piglets would all prove valuable features to decrease cost and labor for producers. These matters further complicate the development of an ideal vaccine.
The need
With increasing restrictions on antibiotic use around the world, more studies on S. suis vaccine development and/or improvement are crucial. Given the vast diversity among S. suis infections by region, autogenous vaccines are likely to be the best option for protection against this bacterium that poses a risk to swine and human health. That said, testing protocol for these vaccines urges to be standardized internationally and more studies need to be performed in order to promptly draw coherent conclusions on the subject – before we lose control of S. suis.



