Several advanced laboratory techniques are nowadays employed to evaluate the cellular immune response in the pig, either using immune blood cells (peripheral blood mononuclear cells, PBMC) or immune cells derived from peripheral organs such as thymus, lymph nodes, spleen, intestine, lung and bone marrow. These techniques have been highly improved in veterinary science and can be employed at present in field or experimental research. Flow cytometry is also used in small animal oncology.
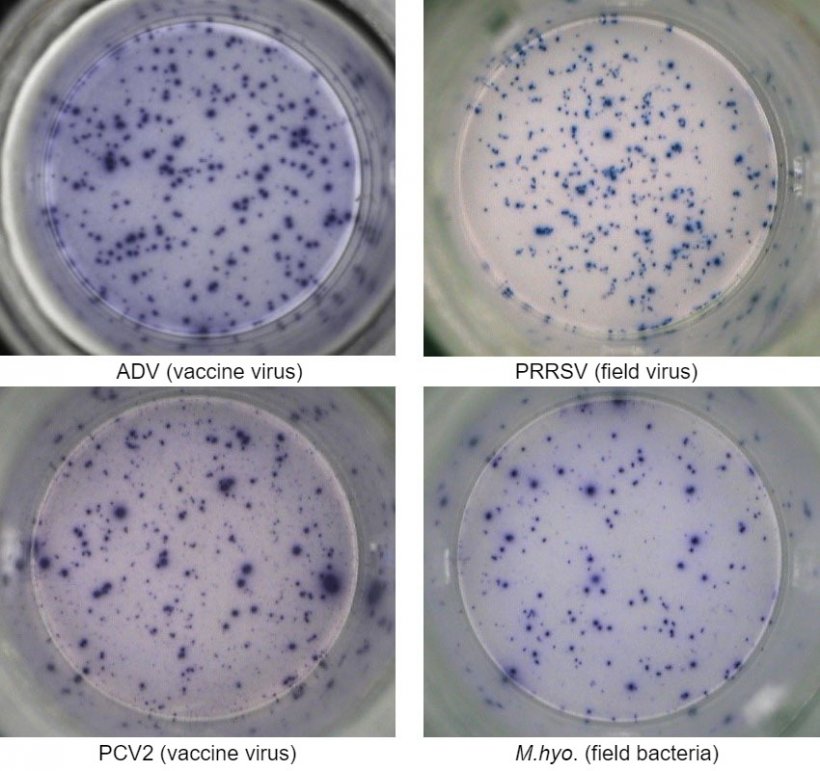
One of the most employed technique is the interferon-gamma enzyme-linked immunospot (IFN-γ ELISPOT) assay by which the number of IFN-γ secreting cells can be quantified with high sensitivity after cell stimulation with a viral antigen (e.g. PRRSV, PCV2, ADV) or mycoplasma/bacterial antigens (e.g. Mycoplasma hyopneumoniae, Escherichia coli).

PBMC isolated from whole blood of animals that came in contact with the pathogen due to previous vaccination and/or natural infection are isolated and tested for quantification of T lymphocytes that are re-activated with an antigen of the same pathogen, or the whole pathogen itself (e.g. whole virus).
This assay can provide relevant information on the effective activation status of the immune system of the animal. In fact, the virus- or bacterial-specific induction of memory and effector T cells is detected by means of the IFN-γ secretion at single cell level visualized in 96-well plates.
By using ELISPOT assay, the immune reactivity can be monitored over time after classical or innovative ways of vaccination (e.g. intramuscular vs. needle-less intradermal delivery), vaccination schedule and also upon infection with new emergent pathogen strains in order to evaluate the response in infected animals and the efficacy of new vaccines in terms of protection and cross-protection.
The cellular immune response is evaluated by enumeration of total spots due to IFN-γ secretion by single cells and generally expressed as number of IFN-γ secreting cells in one million of tested cells. If the sample is positive, it means that the animal has been exposed to the viral or bacterial antigen in the field and therefore has developed memory T immunity that can confer a degree of protection upon a subsequent exposure to the same pathogen and/or cross-protection to pathogen variants.
Flow cytometry is a sensitive and specific technique which allows to identify (immunophenotyping) and quantify immune cell subpopulations in the peripheral blood (PBMC) or cells isolated from immune tissues/organs and subsequently stained using flurochrome-conjugated antibodies reacting with major surface or intracellular immune markers.
Therefore, changes in such subsets can be observed and monitored upon specific animal experimental and/or field treatments such as vaccination or administration of immunostimulants or other drugs, as well as upon experimental and natural infections. Usually, the most employed markers for flow cytometry are the CD (cluster of differentiation) molecules expressed on the surface of immune cells by which several subsets of monocytes (e.g. CD172, CD14, CD16, CD163) and lymphocytes (CD3, CD4, CD8, CD27, CD1, CD2, CD16, CD14, CD25, CD79) can be identified.
In addition, the activation status of these cells can be investigated by intracellular staining of activation-related and secretion molecules such as pro-inflammatory (e.g. IL-1, TNF-α, IL-6) and immnune cytokines (e.g. IL-2, IFN-γ, IL-10) and expression factors (e.g. FoxP3).
Thus, by multiple staining, polyfunctional immune subsets able to simultaneously produce multiple cytokines can be identified and studied upon infection. These seem to be the relevant cells able to regulate and provide clearance and protection against pathogens.
![Fig. 2. Examples of flow cytometry immune cell phenotypes of immune cells quantified as percentage or absolute values [number of cells/ml of blood]) in pig PBMC:
a-c) T lymphocyte subpopulations:
CD3+CD4+CD8- = T helper lymphocytes (Th)
CD3+CD4+CD8+ = memory T cells
CD3+CD4-CD8+ = cytotoxic T lymphocytes (CTL)
d-e) pro-inflammatory monocyte subpopulations:
CD172+CD14+CD16+
CD172+CD16+CD163+
CD172+CD14+CD163+
f-g) CD4+CD25+FoxP3+ = regulatory T lymphocytes (Tregs).](https://www.pig333.com/3tres3_common/art/pig333/13851/flow-cytometry_132769.jpg?w=820&q=1&t=1653979446)
Lymphoproliferation upon in vitro stimulation with mitogenic polyclonal (aspecific) and monoclonal (e.g. viral- and bacterial-specific) activators can be an easy way to evaluate immune T cell reactivity when the animal is subjected to treatments that can alter the response to mitogens or pathogen antigens that tigger proliferation and induce a shift from quiescent to activated proliferating cells (i.e. called lymphoblasts).
Lymphoproliferation can be assessed by an MTT assay (incorporation and color change of a reagent by proliferating cells) but also flow cytometry by quantification of the lymphoblasts (high size cells) and/or incorporation of proliferation fluorescent reagents upon in vitro stimulation for 2-5 days.
Tetramer technology has been employed in pigs for quantification by flow cytometry of memory antigen-specific CD8+ cytolitic/cytotoxic T lymphocytes and, more recently, for quantification of memory antigen-specific B cells.

It is based on the use of antigen-loaded fluorescent macromolecules which are exposed to a mixed immune cell population in order to interact with the B and T cells that selectively recognize the antigen and presumibly are responsible for the efficacy of the cellular response and clearance/reduction of the pathogen amount in the animal body. This is a highly specific and sensitive method that is able to detect minor fractions of memory B and T cells. For instance, it has been developed for recognition of foot-and-mouth disease virus (FMDV)-specific and swine influenza virus (SIV)-specific CD8+ cytotoxic T lymphocytes, and PRRSV-specific memory B lymphocytes.
The cytotoxicity assay employes the use of cytolytic/cytotoxic T effector lymphocytes (E) previously isolated and then incubated for 3-4 days in 96-well plates with target cells (T) at different ratios (E/T); the efficacy of cytolysis provides information on the immune potential of the effector T cells against target cells in vivo potentially infected.





