Introduction
Pigs in a commercial production system are continuously exposed to various pathogens and viruses. Chronic exposure to clinical and subclinical infection compromises intestinal barrier function and activates a systemic response which reduces the rate of protein deposition and growth efficiency. The systemic response influences performance of pigs in two ways: alteration of nutrient partitioning and eicosanoid mediator-induced neurological responses to infection such as anorexia. Role of nutrition to attenuate performance loss caused by activation of immune system will be summarized.

Meeting altered amino acid requirements
Immune system activation decreases feed efficiency by altering nutrient partitioning. Release of pro- and anti-inflammatory cytokines stimulates the liver and increases hepatic uptake of amino acids to produce immune molecules (Breuille et al., 1994; 1998). Significant amino acid catabolism in the skeletal muscle is evident in immune system-activated pigs and rats and depends on severity and duration of the inflammation (Breuille et al., 1994; 1998; Williams et al., 1997a; 1997b). A comprehensive review on role of individual amino acids for whole body protein deposition in immune system activated pigs was published (Rakhshandeh and de Lange, 2011). Briefly, despite increased metabolic demand for glutamine, arginine, phenylalanine, tyrosine and branched-chain amino acids during mild immune system activation, their requirements do not increase due to sufficient de novo synthesis or sufficient amounts present in current diets. Although only limited information is available, it is believed that threonine requirements may be increased in cases where mucus production or threonine-rich immunoglobulin production is stimulated during immune system activation since threonine is the major amino acid in mucus and some immunoglobulins (Faure et al., 2007; Rakhshandeh and de Lange, 2011). Using intramuscular injection of an E. coli lipopolysaccharide (LPS), it was demonstrated that Trp requirement increased by 7% in immune system-activated growing pigs (de Ridder et al., 2012). The sulphur amino acids (SAA), especially cysteine, requirement is known to increase in immune system-activated pigs. Recently a weaner and a grower finisher pig studies were conducted with E. coli infection model and LPS injection model, respectively, to mimic the level of immune system activation experienced in commercial herds, where chronic challenges from pathogens and viruses exist (See Figures 1 and 2). These studies demonstrated that unlike healthy pigs, the rate of whole body protein deposition in immune system activated pigs was decreased by 12% at currently recommended SAA levels (NRC, 2012) and maximum protein deposition was only reached when SAA were supplied at 20% higher levels than currently recommended by NRC (SAA:Lys ratio of 0.75; Kim et al., 2012).
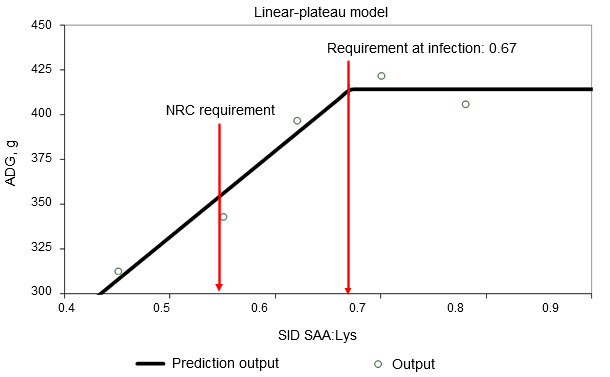
Figure 1. Standardized ileal digestible sulphur amino acid requirement determined in weaner pigs orally infected with an enterotoxigenic strain of E. coli (Capozzalo et al., unpublished data)
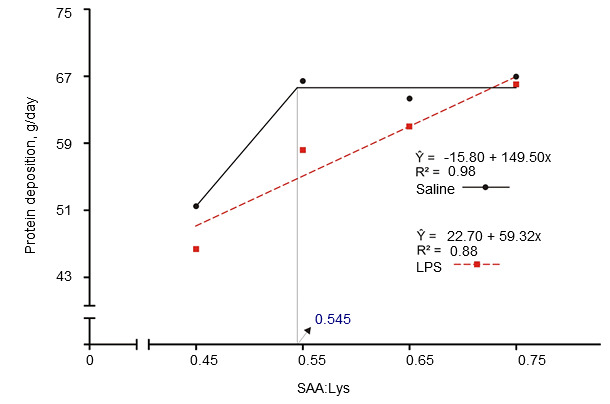
Figure 2. Standardized ileal digestible sulphur amino acid requirement determined in finisher pigs with intramuscular inject of either saline or E. coli lipopolysaccharide (LPS) (Kim et al., 2012)
Manipulating eicosanoid pathway
One of the most important effects of immune system stimulation is increased eicosanoid production, such as prostaglandin E2 (PGE2) and leukotriene B4(LTB4),that induce infection-associated anorexia (Wymann and Schneiter, 2008) and control the duration of inflammation, respectively (Devchand et al., 1996). Production of eicosanoid mediators in the cell and nuclear membranes is initiated through the action of phospholipase A2 or C which respectively convert membrane-bound phospholipids and diacylglycerols to arachidonic acids. Arachidonic acids are then converted to either PGE2 or LTB4 by cyclooxygenase and lipoxygenase, respectively (Folco and Murphy, 2006; Wymann and Schneiter, 2008; Kalinski 2012). Omega-3 fatty acids are known to competitively reduce production of pro-inflammatory eicosanoids by acting as substrates for cyclooxygenase and lipoxigenase enzymes, while antioxidants such as vitamin E and selenium reduces tissue damage by attenuating phagocytosis-associated reactive oxygen species. Boron supplementation reduces inflammatory response by binding proteases produced during the phagocytosis (detailed mechanism can be found in Kim et al., 2013).
Conclusion
Pigs in a commercial environment are consistently exposed to (sub) clinical infections and stressors, and systemic responses to such challenges significantly reduce the growth potential of modern pigs. The systemic immune response influences performance of pigs in two distinctive routes: alteration of nutrient partitioning and neurological response to infection such as anorexia. Supplementation of selected amino acids that are extensively used during the systemic response to counteract the altered nutrient partitioning, and supplementation of nutrients that reduce production of eicosanoid mediators that in turn provokes a neurological infection response, were suggested as possible solutions.

*Aspects of this article were published originally as “Impact of the systemic response to stressors and subclinical and clinical infection on intestinal barrier function and growth in pigs” in “Manipulating Pig Production XIV (eds. J.R. Pluske and J.M. Pluske), pp. 62-76, Australasian Pig Science Association.




