Removing nitrogen from slurry involves transforming it into a gaseous stream of N2 (dinitrogen, an inert gas that composes approximately 78% of the atmosphere). This transformation can be carried out by biological methods, which are the most common, or by physical-chemical methods, such as catalytic oxidation, with limited application and is in the experimental phase for slurry.
Biological removal of ammoniacal nitrogen

In this system, which is applied to the liquid fraction of slurry, ammoniacal nitrogen (NH4+) is transformed into molecular nitrogen (N2). This biological transformation is carried out by combining two processes catalyzed by two different types of microorganisms.
- Nitrification (N): aerobic autotrophic microorganisms, which obtain carbon from CO2 or from the bicarbonate contained in the slurry, convert NH4+ into nitrites (NO2-) and nitrates (NO3-)
- Denitrification (DN): another group of heterotrophic microorganisms converts NO2- and NO3- into N2 gas, using organic matter as a source of carbon and energy.
Figure 1 shows the classic NDN (nitrification-denitrification) system, in which first the denitrification reactor (DN) and then the aerobic nitrification reactor (N) must be arranged, with recirculation to bring nitrates and organic matter into contact in the DN reactor. If it were arranged the other way around, the aerobic bacteria would consume the organic matter and it would not be available for denitrification.
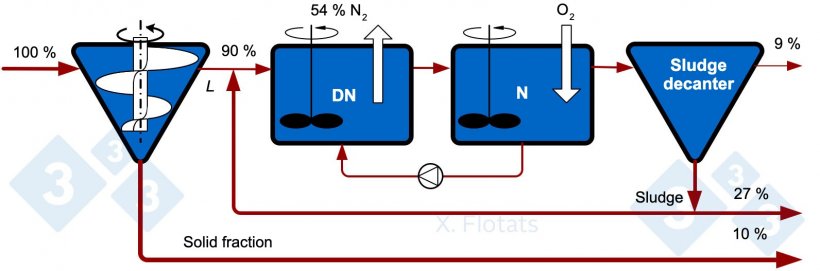
Figure 1. Diagram of the classical NDN system for the biological removal of nitrogen. The indicated values correspond to reference percentages of N distributions assuming a separator efficiency of 10%.
The efficiency of the system is highly variable throughout the year since bacteria (especially nitrifying bacteria) are very sensitive to temperature changes. A well operated system, regulated according to temperature and variations in the composition of the slurry, can have an average nitrogen removal of 60% in the form of N2 with respect to the nitrogen in the liquid fraction separated in the initial S/L separator. It is common for a fraction (around 3-7%) of the nitrogen to escape in the form of NH3 and N2O, which must be prevented.
The system shown in Figure 1 has many possible variations. For example, recirculating the sludge to the header to provide more organic matter for denitrification and recover part of it in the solid fraction; or using an SBR (sequencing batch reactor), in which the two processes are carried out in the same reactor, as well as the decanting of sludge, following an automated time sequence. In this case, the process is discontinuous and a previous buffer tank is necessary to regulate the continuous production of slurry and the discontinuous treatment. Figure 3 shows a diagram of this system, which is simple from a civil works standpoint but more complex due to the need for automation.
An air compressor or a surface agitator is required for oxygen input (see Figure 2), with the consequent electrical consumption. Reducing this consumption can be achieved by operating in such a way that nitrification produces nitrites and not nitrates, and denitrifying from nitrites, also reducing the consumption of organic matter, which is very helpful in slurry due to its low C/N ratio.
One innovation the system has is autotrophic denitrification, by means of autotrophic anammox bacteria, in which they produce N2 from NH4+ and NO2-. This implies that nitrification has to be partial (PN), only converting about 50% of the ammonium to nitrites (even lower O2 and energy consumption), and that no organic matter can enter the process since the bacteria are autotrophic.
If the easily biodegradable organic matter were to reach the anammox reactor, it would facilitate the growth of heterotrophic bacteria, the DN denitrification bacteria, faster than the anammox bacteria. This system has its scope of application when the organic matter has been previously removed by anaerobic digestion and biogas production.
Through biological nitrogen removal technologies, a fertilizer resource is lost, at the cost of consuming energy, which goes against the principles of sustainability and circular economy. For this reason, it is not considered a best available technology (BAT) for new livestock farms. However, it can be considered a technology for emergency plans to combat the nitrate problem until there are solid markets established for the products recovered from slurry.
Ammoniacal nitrogen can also be removed by catalytic oxidation. This system is in the experimental phase and consists of oxidizing ammonia to N2. Depending on the selectivity of the catalyst, or its degree of aging, other forms of nitrogen may be produced, such as N2O, NO or NO2, the emissions of which must be avoided.

Figure 2. Picture of the installation for the biological removal of nitrogen by means of NDN on a swine farm. In the foreground is the denitrification lagoon/reactor and behind it is the nitrification lagoon/reactor, where the aeration system can be seen.
The NDN system in Figure 1 supports extensions to obtain products that allow the export of the remaining nutrients.
NDN combined with composting of solid fraction and sludge, for export

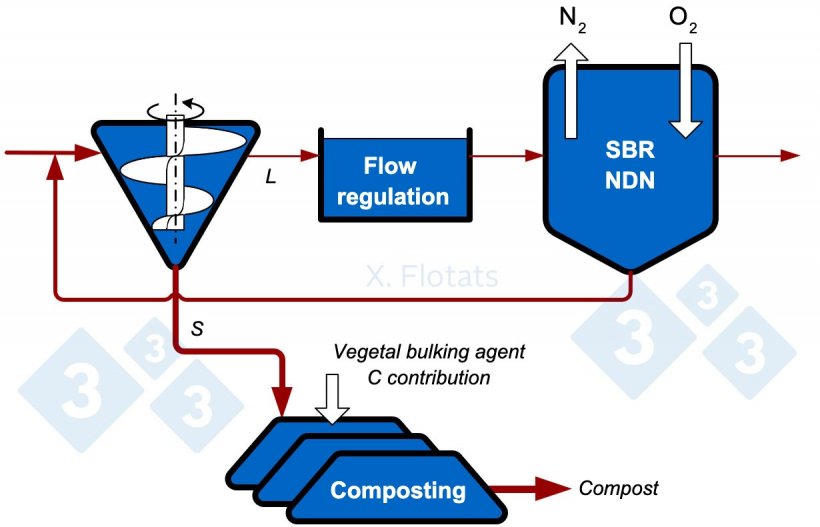
Figure 3. Diagram of a combined NDN system, using SBR, and composting of the solid fraction, with export of the compost and fertigation with the treated liquid fraction.
With the approximate nitrogen distribution percentages in Figure 1, 37% is exported as compost and a remaining 9% is left in the treated liquid effluent, which will be used to fertilize nearby fields.
With almost 10 times less nitrogen, it is possible to make the mistake of thinking that 10 times less agricultural area will be needed. As nitrogen concentration drops, phosphorus or potassium does not necessarily drop by the same magnitude, and either of these elements, or salt or heavy metals, can be limiting factors for application. Fertilization should always be planned according to the most limiting component.
NDN combined with precipitation of phosphorous salts, for export of precipitate and solid fraction, composted or not
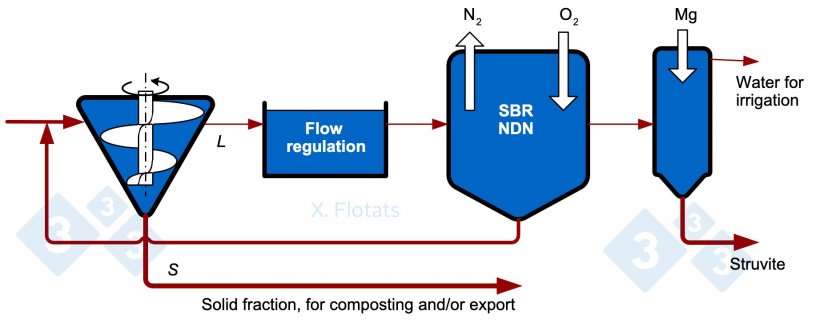
Figure 4. Diagram of a combined NDN system, using SBR reactor, with struvite precipitation, for export together with the solid fraction, composted or not.
If phosphorus is the limiting element after a large part of the nitrogen has been removed with NDN, a phosphorus precipitation unit can be added. Phosphorus can be extracted from the slurry by precipitation techniques. It is recommended to apply these techniques to the liquid fraction obtained from an S/L separation system after having applied some method of reducing the organic matter content, such as NDN or anaerobic digestion, to avoid contamination. A high pH, ≥ 9, is best for the two processes described below which may result in volatilization of NH3, which should be avoided.
Struvite precipitation: Struvite, ammonium magnesium phosphate (MgNH4PO4·6H2O), is considered a slow-release mineral fertilizer and has gained importance as a valuable product in the fertilizer market. The previous elimination of organic matter allows larger and cleaner struvite crystals. Magnesium is found in low concentrations, so the addition of Mg(OH)2 or MgO is necessary. With the N/P ratio of struvite, for every kg of P that precipitates, the N content of the effluent is reduced by 0.45 kg.
Precipitation of calcium phosphates: If no further reduction of the ammonium concentration is desired, the addition of quicklime (CaO) promotes the precipitation of hydroxylapatite (Ca5(PO4)3OH). As with struvite, prior removal of organic matter prevents or reduces contamination of the product and increases its economic value.
NDN combined with separation by membrane of the treated effluent
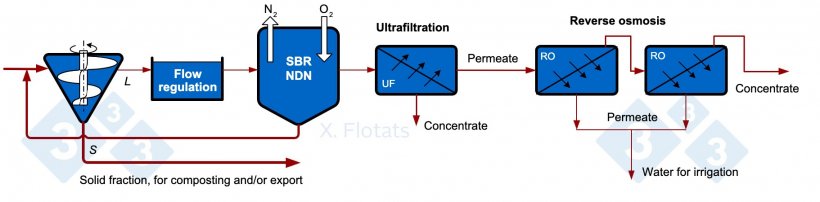
Figure 5. Diagram of a combined NDN system, using SBR, with membrane filtration of the treated effluent, for export of concentrates together with the solid fraction, composted or not.
Membrane filtration processes consist of the separation of particles from a liquid effluent according to their size or salinity by means of semi-permeable membranes. Of the different types of membrane processes, only reverse osmosis can concentrate salts; the others (microfiltration, ultrafiltration, nanofiltration) can only separate particles or molecules of high molecular weight. Reverse osmosis consists of reversing the osmotic flow of water (which would go from a dilute medium to a more concentrated medium) through a semi-permeable membrane by applying pressure, obtaining a permeate (dilute phase) and a concentrate. To avoid contamination, S/L separation and filtration systems must be applied prior to the process; therefore, an ultrafiltration unit has been added in Figure 5. Reverse osmosis can concentrate more than 99% of the salts, but small non-ionized molecules, such as ammonia, can pass through the membrane, so that the water obtained as a permeate is not completely clean.
The benefit of the system lies in being able to valorize the concentrates obtained. In this case, they will have a low concentration of nitrogen (eliminated in the NDN) and phosphorus (mostly separated in the sludge and in the solid fraction), so they will have a low value in the fertilizer market. The final objective, therefore, will only be to obtain water with very low salinity.
I would like to know more about S/L separation systems 
Could solar drying be used to reduce the moisture content of the solid fraction? 
Return to the nutrient surplus situation to study other technological strategies 








