Sperm concentration
Sperm concentration of the ejaculate (number of sperm/ml) is a very important value, because, together with volume of the ejaculate, it will determine how many doses can be prepared. No doubt, this is the most critical and controversial point of the whole process, as there is significant variation on the different measurement equipment, and the lack of a regular and continuous calibration process can lead to the serious mistake of preparing doses with a lower concentration than would be desirable to provide suitable fertility or, on the contrary, with an unnecessarily high concentration, thus decreasing the productive potential of the boar. Currently, porcine AI studs need to produce at least two types of doses: doses for cervical insemination (doses of 80-100 ml) or doses for post-cervical insemination (doses of 30-60 ml). Therefore, high accuracy is required when assessing concentration, especially for post-cervical insemination, as a lower number of sperm will be used (Bortolozzo et al., 2015).

Figure 1. Equipment to measure semen concentration. (a) Haemacytometer: (A) Bürker Chamber for manual cell counting. (B) Colorimeter: electronic measurement through calculation of absorbance. (C) Measure of spermatic concentration directly in diluted semen.
The analytical methods to assess concentration can be manual or automatic. The haemocytometer (manual method: Bürker, Neubauer or Thoma chamber amongst others) (Figure 1a) has been replaced by electronic devices (automatic method) (figure 1b), mainly colorimeters, because they are faster when it comes to calculations (Camus et al., 2011).
Regardless of the method used to assess concentration, errors can be made during the measurement, either because of mistakes in the semen sample preparation methods or because of failure of the measuring equipment. To reduce the possibility of making calculation mistakes, the equipment should be regularly calibrated. Interestingly, to calibrate these electronic devices we still use manual methods such as the haemocytometer. These counting chambers consist only of a glass device, that has a chamber with a known volume where the semen sample to be analysed is introduced. The precision that they offer will depend on the counting area used. The bigger the counting area and the more replicates of the test are run, the smaller the error that will be obtained.
Figure 2 compares the sperm concentration (number of sperm x 106/ml) of semen samples from different ejaculates (1-14) obtained with a colorimeter and the Bürker chamber.
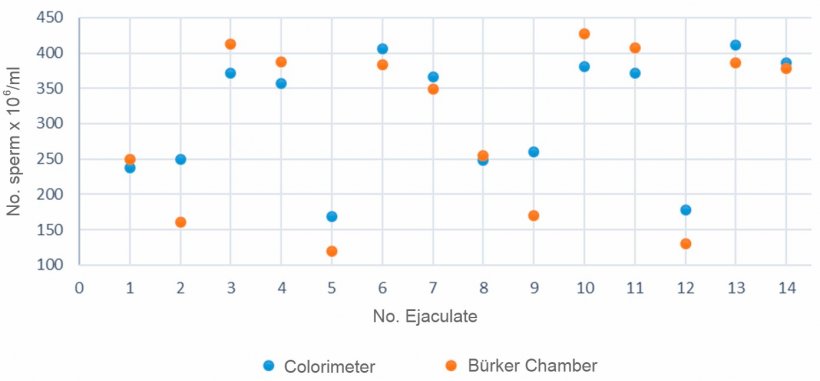
Figure 2. Comparison of the calculated sperm concentration between a colorimeter and the Bürker's chamber for the same semen samples.
Several points in the procedure used to assess concentration can affect the quality of the result. For example, given that sedimentation speed of the sperm is high, it is very important to properly homogenise the collection cup contents when obtaining the sample of the ejaculate to be assessed. If the elapsed time between collection and analysis is longer than 10 minutes, the sperm concentration in the area where sample is pipetted —the upper part of the ejaculate—, will be lower than the real one.
On the other hand, Figure 3 compares the results of sperm concentration when wiping and not wiping the external part of the tip of the automatic pipette. When we introduce the tip of the pipette in the ejaculate to draw in the semen sample, some sperm adheres to the outer walls of the pipette plastic tip. When the pipette contents are ejected in the colorimeter cuvette, this external volume is added to the sample. The results showed that the difference in the spermatic concentration obtained if cleaning or not the external part of the tip of the pipette could be as much as 20% (Figure 3)
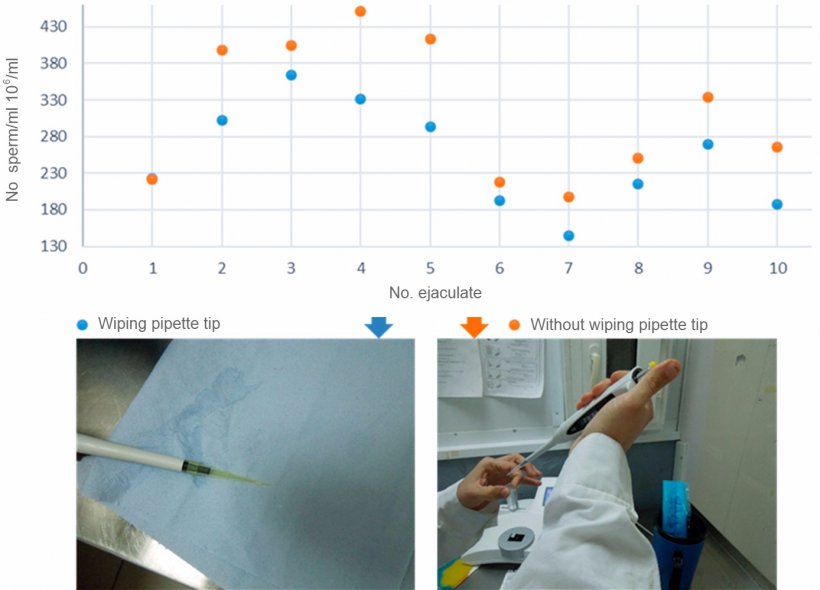
Figure 3. Comparison of sperm concentration between pipettes with wiped tips and non-wiped tips
The final objective of calculating sperm concentration is none other than to guarantee that the semen doses delivered by the AI stud have the minimum spermatic concentration required per dose, with no errors. A simple method that can help us assess spermatic concentration of the semen already diluted, prior to packaging it, is to re-evaluate concentration with a different device, thus assessing the difference between the real and the desired concentration. This process can be very quick to apply using equipment that can read the diluted sperm directly (Figure 1c).
Conclusions
In conclusion, to ensure a maximum quality control of the semen doses prepared in our AI studs, a quality protocol that includes at least the important points mentioned in these articles (Table 1) is undoubtedly required . As a starting point of the semen doses preparation three points should be considered: the correct management of boars, since this will increase the boars productivity and semen quality, adequate facilities and the right management in order to carry out proper semen collection and processing procedures. Once in the lab, all the preparation process will aim to accurately assess the quality parameters of the ejaculate, and to ensure the preparation of top quality semen doses that ensure a high fertility after the AI. Nowadays, the AI stud methodology and protocols are increasingly evolving to the obtention of semen doses with a reduced number of sperm, such as the ones used in post-cervical insemination or fix time AI (Ulguim et al, 2016). In any of these situations, the number of sperm used during AI is being reduced, so proper preparation of the doses is very important to achieve correct fertility rates in the farms.
Table 1. Control points for a correct management and preparation of the semen doses.
| CRÍTICAL POINTS | DESCRIPTION | |
| Doses temperature |
- Mix the semen with the diluent at a temperature slightly lower than the collection temperature (33 ºC) - Leave doses ~100 minutes to reach room temperature (24 ºC) - Store doses at 15 ºC |
|
| Semen Quality |
- Perform an initial evaluation of the semen quality (motility and morphology) - At a regular basis, control and calibration of the automatic motility analysis systems - Perform the analysis using a systematic and methodical approach. |
|
| Spermatic Concentration |
- Homogenize the sample just before the analysis - Routine control/calíbration of the automatic equipment used to measure sperm concentration -To perform the analysis using a systematic and methodical approach. |
|







