Despite the availability and consistent use of vaccines against influenza A virus (IAV) in pigs, the disease continues to be an important economic burden for the swine industry. Vaccines used in swine typically consist of whole inactivated virus (WIV) containing an oil-in-water based adjuvant that is administered through the intramuscular route. Other vaccine platforms have been explored, and a non-replicating alphavirus RNA particle that delivers the HA gene as a subunit vaccine is commercially available for swine in the United States. In addition, farms can implement autogenous vaccines. Vaccination is generally performed in breeding females to provide transfer of maternal antibodies to their litters via colostrum, but grower/finisher pigs are occasionally vaccinated as well. While several fully licensed commercial vaccines are available, they are not updated as frequently as the virus evolves antigenically and can result in suboptimal protection against antigenically distinct strains.
Evidence of safety and efficacy are required for vaccines to be licensed for use in swine. To fulfill efficacy requirements manufacturers must demonstrate immunogenicity of the formulation by hemagglutination inhibition (HI) and/or reduction of virus titers in the lungs of vaccinated pigs experimentally challenged with the homologous vaccine strain virus. The amount of time it takes to modify the formulation of an already licensed vaccine can vary depending on the countries’ regulations, and this hinders much-needed updates of vaccine formulations to match the rapidly evolving virus. However, recent regulatory changes implemented by the USDA Center for Veterinary Biologics may reduce this time in the future.

Vaccine efficacy in the context of field settings should consider each herd individually and the desired outcome. The HI assay, the gold standard for antigenic characterization, typically correlates with virus neutralization titers and is used to infer protection. A reciprocal titer of 40 or higher is generally thought to be protective, however serum HI titers are not always well correlated with vaccine efficacy and protection, in some cases not even against matched virus strains when there are mitigating influences at the time when the vaccine is administered.
Vaccine licensing processes are laborious, costly and can take an extensive time. Ideally, formulation updates for swine IAV vaccines could follow a similar process as that of the annual seasonal human vaccine strain selection based on national and regional surveillance and identification of newly emerged antigenically distinct viral strains (Fig. 1). The challenge remains that the genetic and antigenic evolution of swine influenza viruses is far more dynamic than that of human viruses, with substantial variation observed between different geographical regions and even co-circulation of many distinct strains in a given region (Vincent et al., 2014).
Surveillance and monitoring of circulating IAV strains is the critical foundation for making vaccine decisions (Fig. 1). Analyzing available sequence data of circulating swine viruses and known antigenic positions in the HA amino acid sequences are the first steps to developing a more dynamic and up to date vaccine selection program. Unfortunately, amino acid sequence similarity (and less so for nucleotide similarity) is not always predictive of cross-protection. Major antigenic changes are associated with six amino acid positions near the receptor-biding site of the HA in swine H3 viruses, and even a single amino acid substitution has significant impact on antigenicity (Lewis et al., 2014). Swine H1 viruses, on the contrary, have more complex genetic diversity, possibly as a result of evolution of classical viruses and repeated introduction of human viruses into pigs over a longer period of time. Consequently, antigenic changes have not been attributed to single amino acid mutations within H1 hemagglutinins, although substitutions in or near the receptor-binding site have an important cumulative effect in determining antigenicity. Hence, antigenic characterization is a crucial step that should be used in combination with sequence analysis and epidemiological information to better inform on vaccine decision-making (Fig. 1).
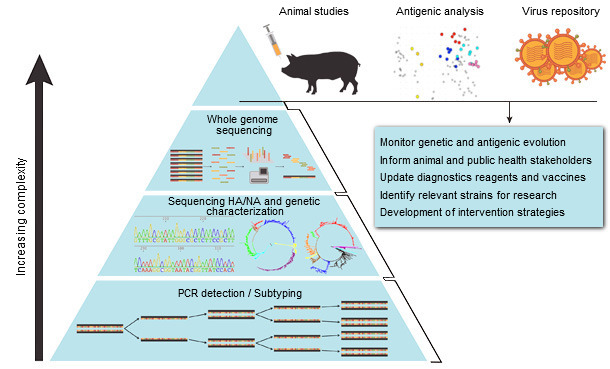
Figure 1. Systematic approach to influenza A virus surveillance in swine (based on Ampofo et al., 2015).
Under ideal circumstances, the immune response elicited after killed virus vaccination is sufficient to reduce clinical signs and lung viral titers after infection, provided that the challenge virus is antigenically very similar. However, inactivated vaccines often do not completely block infection under typical field situations. Maternal derived antibodies elicited by vaccination of sows can protect piglets against clinical signs but not against infection, and can be detected up until 14 weeks of age (Loeffen et al., 2003). A detrimental effect of these antibodies is that they can interfere with vaccine efficacy and the active immune response of a vaccinated piglet. In fact, prior immunity, whether passive or acquired, can interfere with immune responses to killed vaccines.
Antibodies stimulated by killed virus vaccines or passive transfer are unlikely to provide complete cross-protection against infection with a mismatched virus, resulting in vaccine failure and increased expenses for producers. Vaccine failure can be further complicated if vaccination induces cross-reactive antibodies to an HA of the same subtype that fail to neutralize the subsequent challenge virus (i.e., not cross-reactive in the HI assay), leading to vaccine-associated enhanced respiratory disease (VAERD;(Gauger et al., 2011)). For VAERD to be manifested, a mismatch between the HA and NA of the viruses in the vaccine and the infecting virus is necessary. VAERD has been demonstrated in many experimental scenarios, and there are indications that it occurs in the field.
The limitations for inactivated vaccines to provide cross-protection against antigenically different viruses highlights the need to develop more broadly cross-protective vaccines. Various vaccine platforms for IAV have been evaluated in swine with each having strengths and weaknesses (Fig. 2). Live-attenuated IAV vaccines (LAIV) have been repeatedly demonstrated to be safe under experimental conditions and more efficacious against antigenically distinct viruses (Fig. 2), reducing viral transmission, and overcoming interference by maternal antibodies (Pena et al., 2011; Vincent et al., 2012). Protein subunit and vectored RNA or DNA vaccines targeting the HA and NA have also been shown to protect against infection, and one advantage of these newer technologies is the relative ease in modifying the vaccine formulation to account for antigenic diversity and novel emerging viruses (Wesley et al., 2004). Novel vaccine platforms will continue to be explored as alternatives to killed virus vaccines.
 Whole inactivated virus |
 Vectored |
 Live-attenuated IAV vaccines |
|
| Delivery route | Intramuscular | Intramuscular/Intranasal | Intranasal |
| HI response | +++ | ++ | + |
| Antibody secreting cells | ++ | + | + |
| Memory B cells | + | + | + |
| Nasal IgA | -/+ | -/+ | +++ |
| NA antibody | +++ | -/+ | ++ |
| CD4 T cells | ++ | ++ | +++ |
| CD8 T cells | - | + | + |
| Cross protective immunity | -/+ | + | ++ |
| Vaccine-associated enhanced respiratory disease (VAERD) | yes | -/+ | no |
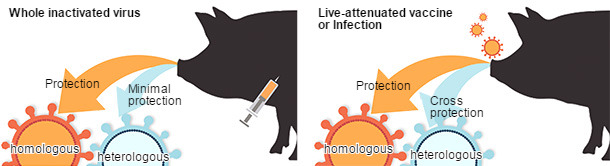
Figure 2. Comparative efficacy of vaccine platforms against influenza A virus infection in swine (based on Sridhar et al., 2015).
Vaccine strategies should be considered on a case-by-case basis and in the appropriate context. Inactivated vaccines can be effective if used in conjunction with other practices, such as controlled movement of animals and people and with careful analysis of whether the vaccine antigen is a good antigenic match with the circulating strain. Knowledge of virus strains circulating up- and downstream of each farm is also necessary. Swine influenza is a dynamic disease that continually evolves, thus monitoring and control strategies need to be intensive and responsive to keep pace.




