The use of antibiotics in both human and veterinary medicine is in the spotlight due to the threat posed by the emergence and development of resistance. The European Union has faced the challenge with its own strategy, which results are beginning to be seen. What will happen to antibiotics in the European Union? Let us try to review the current situation and what is coming.
Regulations under development

EU legislation on veterinary medicinal products and medicated feed is currently being reviewed.
Experts have been working on the proposal for the regulation of veterinary drugs since 2014. At the same time, the regulatory proposal for the amendment of the regulation on medicated feed —which will affect its manufacture, launching and use—, is being revised.
Antimicrobial resistance (AMR) is, globally, a serious problem. Irresponsible use of antibiotics in human and veterinary medicine can contribute to the development of AMR. These new normative proposals derive from the Commission's Strategy to fight this threat, being the measures to control this problem very much on the agenda. This debate confirms the need for a clear scientific approach to be able to establish realistic and effective measures.
With regard to the regulation of veterinary medicines, the debate focuses on:
- Marketing authorization procedures: Establishing a centralized authorization procedure would increase product availability by harmonizing the evaluation process.
- Protection of technical documentation: Extending the period of registration protection will encourage innovation and development of new products.
- Labelling: Promoting mandatory information at European level on the uses of veterinary medicinal products
- Pharmacovigilance: Simplifying and harmonising procedures.
- Off-label uses: They should be considered, especially for the smaller species to have access to products.
- Distribution: The question arises whether it is necessary to harmonise and standardise the conditions of all the operators of the EU distribution channel.
In medicated animal feed, the main points of the debate include:
- Purpose of the proposal: Whether it should only consider medicated feed or extend it to any product given orally.
- Definitions: Many definitions must come from the Veterinary Medicines Regulation, therefore, both regulations should be progressing in parallel.
- Composition and homogeneity. Referring to the use of more than one drug premix and how to manufacture it to ensure a homogeneous presence of the active ingredient.
- Transfer / Cross Contamination: Both referred to the presence of traces of an antibiotic in a feed that should not contain it. The manufacturing process itself makes it technically impossible to avoid cross-contamination 100%, which is why a tolerable maximum must be established which, according to the European Commission, would be 1% of the amount used in the target feed; according to the Parliament, however, this maximum should be 3% and the Council proposes to establish maximums per molecule in mg / kg
- Prescription: Diagnosis should be made prior to prescription. Prophylactic uses should be abandoned in favour of therapeutic and metaphylactic uses.
- Maximum duration of treatment: A maximum treatment length must be standardised in order to avoid routine preventive uses.
- Removal of remaining medicated feed: For the Commission, any medicated feed not consumed should be removed from the holding; for Parliament, it could be used under a number of premises.
Control of antibiotic consumption
One of the main issues is the excessive use of antibiotics. To find out its real volume, a voluntary participation report is drawn up annually to collect the volume of antimicrobial sales in the EU, the ESVAC (European Surveillance of Veterinary Antimicrobial Consumption) report, which contains data from 2010 (19 countries), 2011 (25 countries), 2012 (26 countries), 2013 (26 countries) and 2014 (29 countries).
The evolution of total sales in tonnes of active ingredient and mg/PCU of the countries participating in the ESVAC report can be seen in the following tables.
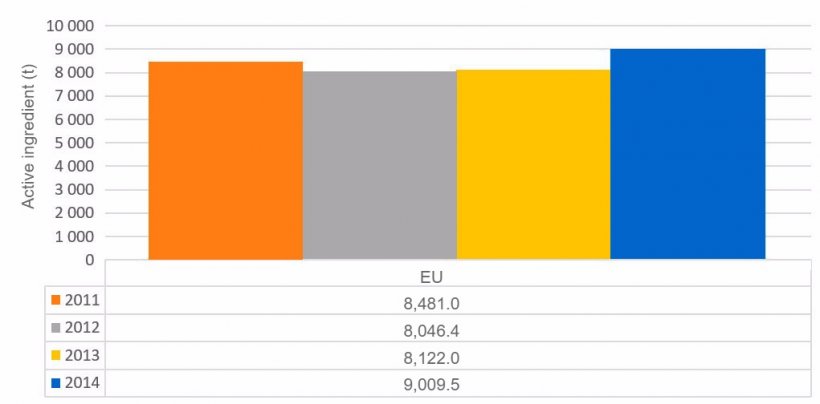
Graph 1. Evolution of total antimicrobial sales in the countries analysed in the ESVAC report.
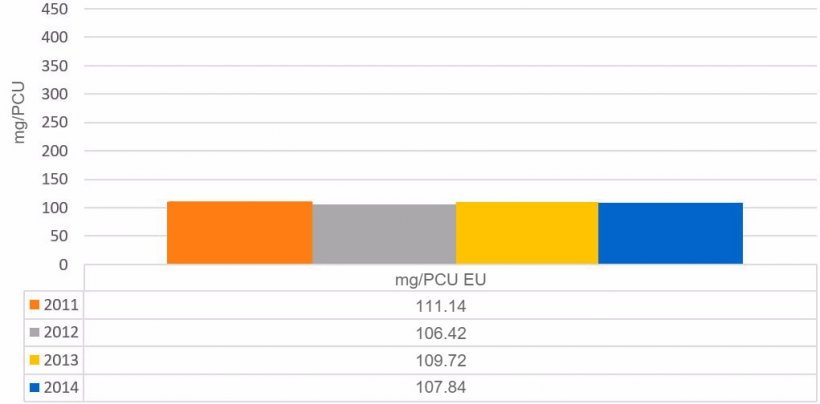
Graph 2. Evolution of antimicrobial sales in mg / PCU in the countries analyzed in the ESVAC report.
Regarding the consumption of antimicrobials per PCU unit, the average for the countries included in the ESVAC 2014 report was 107.84 mg/PCU.
The mg/PCU unit is a system developed to allow for a valid comparison of the use of antimicrobials between countries by comparing the mg of active ingredient sold for the domestic market of that country, with the units of animal product produced. In the case of swine, the Population Correction Unit (PCU) is obtained from the sum of the average census of breeding stock and animals produced for slaughter, estimating an average weight of 240 and 65 kg respectively. These data must be corrected by subtracting or adding, the import and/or export of piglets and/or pigs for slaughter.
Current normative review

EU legislation on veterinary medicinal products, including antibiotics, aims at ensuring a high level of health protection and promoting the functioning of the internal market, while helping to encourage innovation. The most important ones are:
- Directive 2001/82/EC on the Community code relating to veterinary medicinal products.
- Regulation 726/2004 on Community procedures for the authorization and control of medicinal products for human and veterinary use.
- Regulation 470/2009 on Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin.
Conclusion
The fight against antimicrobial resistance has put in focus the consumption of antibiotics in particular, and of antimicrobials in general. In line with the European Commission's strategy to fight resistance, the rules on veterinary medicines and medicated feeding stuffs will be modified. The aim is to make a responsible use of antibiotics with a double aim: to reduce, if possible, antimicrobial resistance and, on the other hand, to have effective antibiotics for a long time. Antibiotics are and will be necessary, therefore an effort must be made to preserve them.



