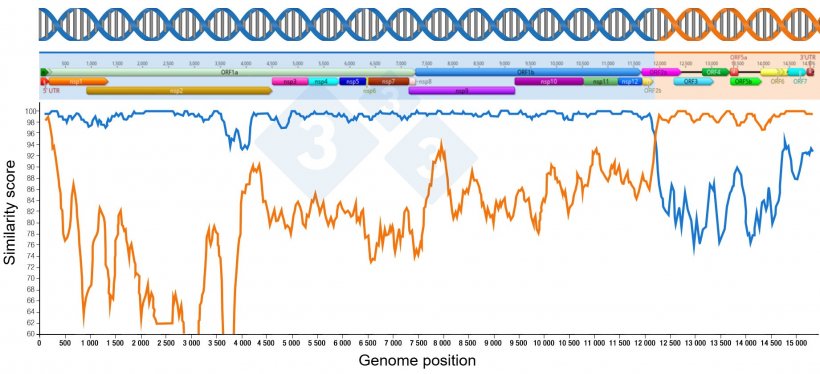
Recombination is a natural occurrence and a driver of PRRSV genetic evolution. The occurrence of recombination has been reported in laboratory cell culture experiments since the late 1990s. For a recombination event to happen, two viruses must infect a cell and replicate, exchanging genetic materials between them and deriving a new progeny virus (Figure 1).

In recent literature, recombination has been reported to have occurred between:
- Wild-type viruses
- Modified Live Vaccine (MLV) viruses
- Between wild-type and vaccine viruses.
Recombination events may have always occurred in the field, but what has changed in recent years is our improved ability to detect such events through the increased use of Next Generation Sequencing, NGS (as explained in the article published on pig333.com on 2 June 2025 “PRRS Molecular diagnostics: when sequencing only 4% is not enough”) and the availability of bioinformatics tools for recombination analysis.
Many recombination events that occur in the field may generate nonviable viruses or viruses that fail to establish stable infection and persist in the population. The larger animal health concern occurs through the recombination of two different wild-type viruses (Figure 2).
The recent emergent Rosalia and L1C.5 strains are examples of aggressive wild-type recombinant PRRS viruses that have endemically established themselves in the swine population and caused aggressive clinical signs.
However, the question that remains is:
- Why there is a perception of more MLV vaccine virus-derived recombinant PRRSV?
- What happens with this new virus?
MLV label recommendations are for usage in healthy animals for developing individual immunity before a wild-type infection. In the wild-type PRRSV battle, MLV viruses often co-circulate with wild-type viruses. Within MLV-derived, recombinant viruses are not as aggressive as wild-type viruses or wild-type recombinants (Figure 2).
In the United States, case reports describing the adoption of measures such as reinforcing biocontainment and biosecurity practices to avoid the spread of the virus across farrowing rooms and improve individual piglet immunity have demonstrated promising results in reducing the clinical implications of these newly emerged PRRSV-2 MLV-derived viruses. In Europe, the report of PRRSV-1 MLV-derived recombinant has also been reported as not being as mild as the MLV virus nor as aggressive as the wild-type virus under clinical experiments.
PRRSV-2 recombinant chimeric viruses produced in the laboratory between wild-type and MLV vaccine viruses and experimentally inoculated in pigs under experimental conditions have been reported to have less aggressive clinical signs compared to wild-type viruses, but have not demonstrated clinical effects as mild as the parental MLV vaccine virus (Figure 2). Additionally, there are also reports where field-recovered wild-type recombinant viruses between MLV vaccines and field strains have posed additional challenges for cell culture growth.
Figure 2: Clinical implications and bioinformatics ability to detect recombination events between different PRRSV.
| PRRS strain | Clinical implications |
Bioinformatics ability to detect recombination |
|---|---|---|
| Modified live virus (MLV) |  |
 |
| Recombinant MLV & MLV | ||
| Recombinant MLV & wt-PRRSV | ||
| Wild-type PRRSV (wt-PRRSV) | ||
| Recombinant wt-PRRS & wt-PRRSV |
Another approach that has been used in some countries to manage PRRSV is the implementation of live virus inoculum (LVI), which involves collecting samples (such as serum or lung) directly from the field, confirming the presence of PRRSV by RT-PCR, diluting the samples in a media, and exposing the animals to the field virus that is present on the farm.
Field viruses are not attenuated, like MLV, and notably, LVI could unknowingly contain multiple strains of multiple PRRSV strains, creating an opportunity for wild-type recombination
Additionally, LVI material usage is not legally approved in many countries and can contain other pathogens circulating in donors' bloodstream, e.g., porcine circovirus, porcine parvovirus, etc., posing additional challenges for disease control.
What are the implications of having multiple PRRS strains and/or recombinants?
- Breeding herds infected with ≥ 3 PRRSV strains had 1,837 higher piglet losses per 1,000 sows compared to herds infected with ≤ 2 strains, which supports the fact that the more PRRSV strains circulating in the population/herd/pigs, the greater the clinical impact/signs (the more = the worse);
- Breeding herds that had ≥ 3 PRRSV strains detected during the PRRSV elimination took 12 weeks longer to achieve stability when compared with herds that had ≤ 2 strains;
- Herds that identified a PRRSV recombinant virus at the time of outbreak had 1,827 higher piglet losses per 1,000 sows compared to farms with no recombination events detected.
Controlling PRRS can help reduce the occurrence of recombination events
Some examples are:
- Avoid introducing gilts that are infected with a distinct PRRSV from the one currently present in the breeding herd;
- Avoid commingling nursery and finishing pigs infected with different wild-type PRRSV
- Test LVI samples by NGS before injection and avoid using LVI material containing multiple genetically distinct PRRSV
- If LVI material has an MLV-like virus, use the MLV virus extracted from a bottle for vaccination purposes instead of a field virus.
- When using MLV, avoid mixing or injecting two MLVs simultaneously. Also, avoid rotating MLV vaccines within a herd or production flow
- Properly follow MLV label recommendations for use in healthy animals to induce individual immunity before an expected wild-type infection.
PRRSV is already a challenging animal health-threatening agent and keeps genetically evolving over time. Controlling PRRSV not only leads to better productivity outcomes but also reduces the opportunities for different wild-type viruses to recombine and create more aggressive ones.





