Porcine reproductive and respiratory syndrome virus (PRRSV) along with African swine fever virus is one of the most economically important pathogens affecting the swine industry globally. The recent emergence of aggressive PRRSV strains like the Highly Pathogenic PRRSV (HP-PRRSV) in Asia, Rosalia in Europe, and the L1C.5 in North America has ignited the discussion on the need to further improve PRRSV diagnostics and control.
The use of RT-PCR for detecting PRRSV genetic material is commonly used worldwide to screen populations for the virus.

One step beyond RNA detection is PRRSV genetic sequencing, commonly done using the Sanger technique. Globally, the portion of the virus most commonly used for PRRSV sequencing is ORF5, although some laboratories report ORF7 sequencing.
The ORF5 represents about 4% and ORF7 2.4% of the PRRSV genome, and thus, these do not provide full genetic coverage of the whole genome.
In recent years, there has been a growing interest in the use of next-generation sequencing (NGS) for the recovery of whole PRRSV genomes for PRRSV epidemiological investigations within a herd or production system (Figure 1).
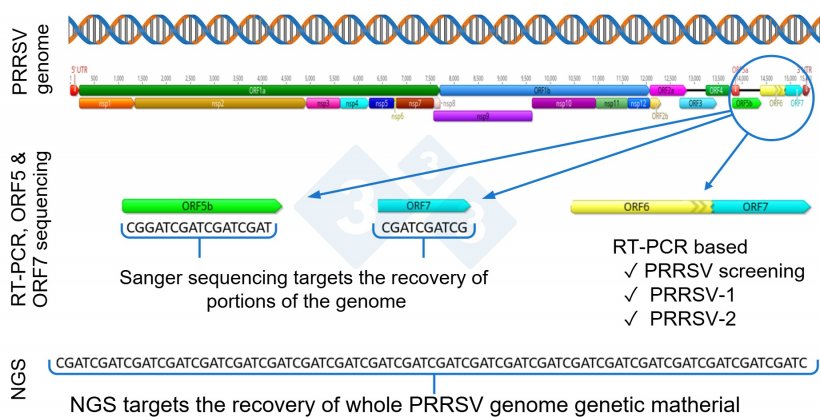
Normally, during the replication process, PRRSV goes through genetic changes and mutations, that may potentially lead to the emergence of new viral variants. PRRSV is one of the viruses known to have a high rate of mutations, about 0.5 to 1% a year, unevenly distributed across different genome regions, virus type, and genetic lineages, leading to constant genetic evolution. Notably, genetic mutation and evolution of the virus can occur across all genes, and sequencing only a portion of the genome, e.g., ORF5 or ORF7, likely misses the opportunity to detect changes that occurred outside the sequenced region (Figure 1).
Under this scenario, NGS becomes a useful tool by providing an opportunity to recover a whole PRRSV genome to be used in epidemiological investigations.
How can veterinarians and producers make the advantage of NGS
To maximize the utility of NGS, there are some points to consider:
- Early involvement of the veterinarian and laboratory diagnostician is important to align the diagnostic question, expectations, and approach for testing.
- Is there a PRRSV referent strain whole genome sequence on a farm or production system for comparison?
- If not, use NGS on PRRSV RT-PCR positive samples with low Ct values, i.e., ideally < 24, to recover a whole genome as the herd-referent strain. The Ct value is inversely related to the amount of genetic copies of the virus present in the sample, i.e., the lower the Ct, the higher the expected viral load and potential success of NGS;
- A referent strain allows subsequent comparison of prospectively recovered genomes to understand PRRSV genetic evolution within the farm, flow, and system.
- What is the objective of doing NGS?
- If the objective is to detect virus evolution at the whole genome level: target lung and serum since these samples are more likely to recover a whole genome.
- If the objective is to understand the viral diversity in a farm or herd, use population sample types like processing fluid, oral fluid samples, or pooled individual samples, e.g., pooled serum is more prone to recover multiple viruses if present in the sample.
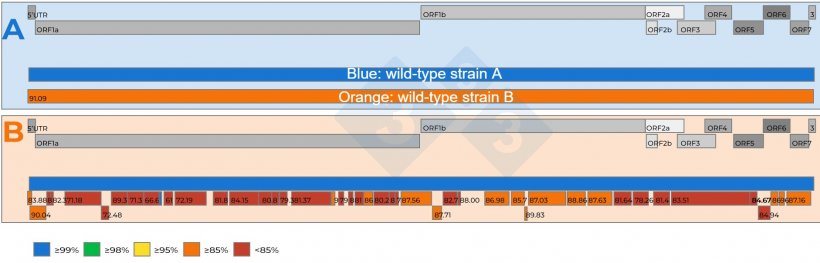
- Mixed infections with two or more PRRSVs in a herd are a reality (Figure 2a), and if the two viruses are very similar, the NGS may fail to recover a whole genome;
- If multiple viruses are present in the sample, NGS may recover genome fragments, referred to as ‘contigs’, from different viruses, which, when compared to a farm reference strain, can help discern if multiple viruses are present in the sample (figure 2b);
- Lastly, be sure to have someone to help with the genetic analysis and comparisons, and coordinate the turnaround time, as NGS is typically a slow process that can take over a week to complete.
Which kind of epidemiological information can we get from NGS
Generated NGS outputs can help sort out questions:
- Did a virus evolve through random nucleotide substitutions at the same genomic positions? How much has the virus changed between two time points, and in which genome, i.e., ORF region(s), did these changes occur?
- Did the virus evolve through insertions or deletions in its genome?
- Did a new introduction of an unrelated virus occur in the farm, herd, or flow?
- Although less likely but possible, did a new virus acquired changes in the genomic regions targeted by RT-PCR or Sanger primer/probe sequencing, causing the assays to fail in detection?
- Did the virus undergo recombination, i.e., acquired some genomic regions from two or more parental viruses
Recombination is a natural process of PRRSV evolution and occurs when two PRRSV replicate in the same cell generating a third derived virus. Recombination will be further explored in article published on pig333.com on 9 June 2025 “The implications of the PRRSV recombination dilemma”.





