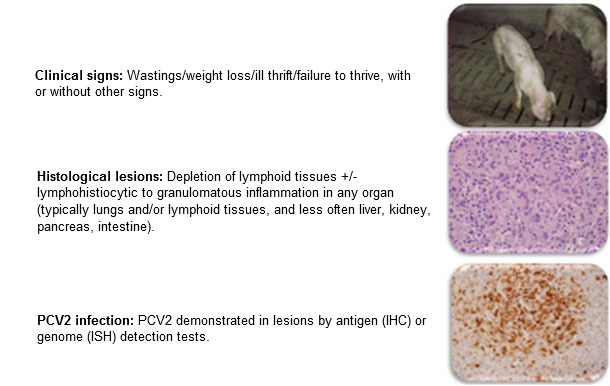
The occurrence and impact of porcine circovirus type 2 (PCV2) infection and disease (PCVAD, formerly PMWS) has changed considerably over the last 15-20 years. Prior to 2004, PCV2 was highly prevalent in pig populations but often asymptomatic, hence confidence in diagnosis of a disease associated with this agent was often lacking. The case definition and diagnostic criteria for diagnosis of postweaning multisystemic wasting syndrome (PMWS, aka. PCVAD) was summarized by Sorden in 2000 (figure 1) and became widely accepted by the veterinary community as this disease spread around the globe and was experienced first-hand. Sorden wisely incorporated typical clinical signs, typical lesions, and demonstrable virus into the case definition and diagnostic criteria.
Figure 1: PMWS: Case definition. Sorden, Swine Health Prod 8(3):133-136, 2000

Diagnosis in a pig or group of pigs requires:
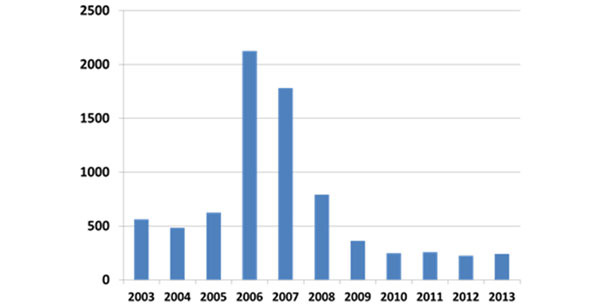
During the epidemic phase (2004-2007), death losses from PCVAD of grow-finish pigs sometimes approached 40%, with overall marketing shortfalls of nearly 10% in the US. By early 2006, producers were desperate for effective interventions. Although various research groups had encouraged commercial vaccine development since the late 1990’s, no commercial product was available in the US until 2006 and it was not widely available until 2007-8. Commercial vaccines proved to be remarkably efficacious, both in preventing devastating losses from deaths and wasting as well as by increasing overall performance and decreasing the variation in weight gain. The uniformly successful intervention with effective commercial vaccines to prevent death loss and wasting continues to date, with disease diagnosis trends illustrated in figure 2.
Figure 2: Relative frequency of diagnosis of PCVAD (ISU VDL)
Vaccines currently remain efficacious for PCVAD and nearly all commercially produced animals in the US are currently vaccinated but, because PCV2 does continue to circulate in vaccinated populations, there are occasions where PCVAD still occurs.
Diagnostic tools and PCV2 variation
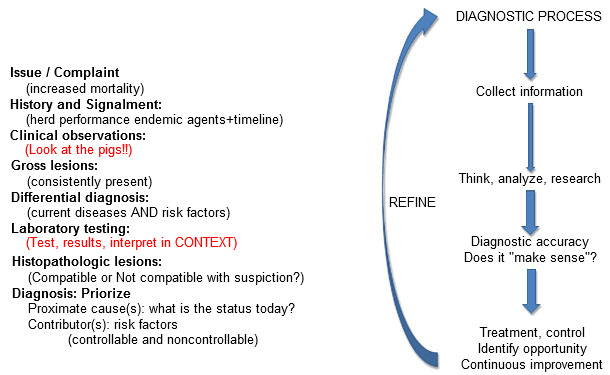
Diagnostic tests are "tools" to be wielded by those with the training and experience in test interpretation within the context of the clinical scenario at hand as well as the gross and microscopic pathology. Confidence in the accuracy of a diagnosis requires that these all "align"- that is, they are accurate and tell a consistent story. Observations or test results that do not make sense should result in immediate re-evaluation of the diagnostic process (figure 3). This is simple in concept but can be easily overlooked in practice. Assumptions, confirmation bias and superficial consideration of diagnostic options can (and often do) lead to erroneous diagnostic conclusions.
Figure 3: Diagnostic "alignment": All information should make sense
Essential diagnostic testing tools for PCVAD are:
- Histopathology with antigen or agent detection within tissue sections (immunohistochemistry, in situ hybridization); and
- Genome detection (PCR) and characterization (sequencing) using molecular techniques.
Immunohistochemistry is an excellent method to demonstrate viral antigen within lesions in histologic sections. However, IHC is not particularly sensitive to detect presence of virus and is also subjective in interpretation. Discrepant results can and do occur. IHC is a tool to be wielded by trained pathologists and when diagnostic alignment is not present, should be scrutinized for accuracy.
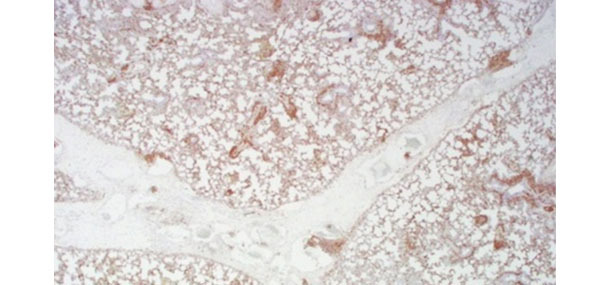
Positive (brown) staining for PCV2 by immunohistochemistry in lung
from a pig with severe interlobular edema and diffuse interstitial pneumonia.
Polymerase chain reaction (PCR) is undoubtedly the most sensitive method for detection of respective specific gene targets. A positive PCR test does NOT confirm disease causation – it simply answers the question "Is PCV2 present in the sample tested?" Quantitative and semiquantitative PCR for PCV2 in serum of pigs is frequently used as a measure of "virus load". The idea that the magnitude of viremia or virus load of a tissue is correlated with disease expression and outcome of infection has proven merit. After infection with PCV2, pigs that do not develop clinical disease will generate virus-neutralizing antibody and the viremia will cease by 3 weeks after infection and the viremia in these pigs will not likely exceed 107 genomic copies per mL, often less. Clinically affected pigs often have values that exceed this number and persist for longer periods. Results will vary amongst laboratories therefore it is incumbent upon swine veterinarians to be familiar with the tests and result interpretations that are unique for each laboratory.
One of the limitations of PCR is that the primers and probes may not detect variants of target virus. This was evident in a recent investigation of a novel "mutant" PCV2b (referred to as mutant PCV2b, mPCV2b or PCV2d) which was isolated from a herd reported to be experiencing "vaccine failure". The ORF2 PCV2a/b differential PCR routinely used by the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL) did not detect a novel PCV2 present in clinical specimens, yet the PCR designed for ORF1 probe was positive. In this situation, IHC applied to tissues was positive for PCV2 (Opriessnig). The idea that PCR probes must be updated is not new and has been fairly common with agents such as PRRSV. Observations or test results that do not make sense should be re-evaluated. Without such feedback from astute veterinary practitioners, no diagnostic laboratory can stay current. The impact of strain variation on performance of serology tests is yet to be determined.
Detection of antibodies is usually by ELISA or serum neutralizing techniques. Because of widespread infection, serology has been of limited value for diagnosis and results should be interpreted with care. Serologic profiling of herds can help determine when infections are occurring or can be useful to assess potential impact of maternal antibody on timing of vaccination(s).
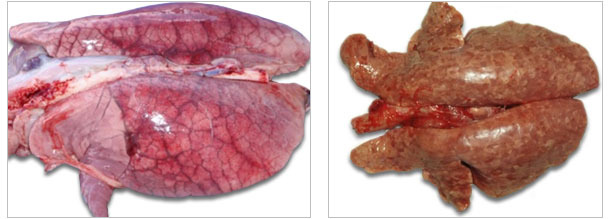
| Acute pulmonary edema syndrome sometimes seen with acute PCVAD. |
Tan and firm lungs (interstitial pattern of pneumonia) from pig with PCV2-associated pneumonia. |
Diagnosis vs assessing herd impact
Diagnosis of the role of PCV2 in a diseased animal remains the same as Sorden described 14 years ago. Finding an occasional pig with PCVAD within a large population is not unexpected since vaccination compliance is rarely 100%. Automatically ascribing all of a population’s performance variation, morbidity or mortality to PCV2 on the basis of observations in a couple of pigs would be imprudent when other endemic pathogens (e.g. bacteria, viruses, parasites) or other factors which compromise performance (e.g. nutrition, management, environment) are also present. A difficult question to answer is: "How does one assess impact of PCVAD in a vaccinated population?"
Systematic approach to diagnosis involves thoughtful, systematic monitoring of pigs over time. Often, simultaneous monitoring for roles of other endemic agents (e.g. Mycoplasma, PRRSV, swine influenza, Actinobacillus, Haemophilus, Lawsonia, Brachyspira, Salmonella, parasites, and more) is more objective and rewarding than simply focusing on PCV2 detection. Probably the most insightful diagnostic tool is systematic necropsy and recording of findings of all deaths in grow-finish phase over time. Tissues can be collected in formalin for evaluation if diagnosis is in doubt or one wishes to rule out a role for PCV2. Often, endemic pneumonia, enteritis or chronic serositis are found to significant contributors to losses. Routine slaughter checks can augment this activity. Serologic profiling can help identify stage or age at which agents are being transmitted. The impact of the multiple factors identified can be ranked and perhaps even be estimated against the total opportunity lost from production potential. Unfortunately, there are as yet no consistent or proven accurate protocols for assessing the impact of PCVAD or subclinical PCV2 infections in populations short of carefully designed and controlled prospective on-farm trials.