Porcine reproductive and respiratory syndrome (PRRS) is an economically significant disease of swine, estimated to cost the US industry approximately $560 million US per year. Clinical outbreaks of PRRS were first reported in the late 1980's in the US; however, the etiology of the disease remained unknown. Similar clinical outbreaks were reported in Germany in 1990 and were widespread throughout Europe by 1991. In 1991, the etiologic agent, porcine reproductive and respiratory syndrome virus (PRRSV) was identified by investigators in the Netherlands and the United States. Today, PRRSV is endemic to the global swine population; however, several countries, including Sweden, Switzerland, New Zealand and Australia claim to be free of the disease.
Etiology
The PRRSV is an enveloped, single-stranded positive-sense RNA virus, approximately 50-65 nm in diameter that is classified in the order Nidovirales, family Arteriviridae, genus Arterivirus along with equine arteritis virus, lactate dehydrogenase-elevating virus of mice, and simian hemorrhagic fever virus. Properties of these viruses include the ability to induce prolonged viremia, persistent infections, and replication in macrophages. Being an enveloped virus, PRRSV survivability outside of the host is affected by temperature, pH and exposure to detergents. PRRSV can survive for extended periods of time (>4 months) at temperatures ranging from -70°C to -20°C; however, viability decreases with increasing temperature. Specifically, recovery of PRRSV has been reported for up to 20 minutes at 56°C, 24 hours at 37°C, and 6 days at 21°C. The PRRSV remains stable at pH ranging from 6.5-7.5; however, infectivity is reduced at pH levels below 6 or above 7.65. Detergents are effective at reducing infectivity of the virus and lipid solvents such as chloroform and ether are particularly efficient at disrupting the viral envelope and inactivating replication.
Regarding genetic diversity, there are two major prototypes of PRRSV, the European isolate (Lelystad virus) and the North American isolate (VR-2332). In addition to differences between isolates, it has been determined that there is ample genetic variation within both isolate types, as confirmed by analysis of the nucleotide and amino acid sequences of the open reading frame (ORF) regions of LV and VR-2332. Amino acid sequences for VR-2332 as compared to LV are 76% (ORF 2), 72% (ORF 3), 80% (ORFs 4 and 5), 91% (ORF 6) and 74% (ORF 7), and sequence analysis indicates that viruses are evolving by random mutation and recombination. Recently, reports of highly virulent isolates in Asia (pig high fever disease) have been reported. These isolates, in combination with other pathogens, such as Classical swine fever have resulted in elevated levels of mortality in China and Vietnam swine herds.
Clinical manifestation
Outbreaks of PRRS involve episodes of reproductive failure (third trimester abortions, premature parturition, and elevated levels of fetal, i.e. mummies and stillbirths and neonatal death) as well as reduced growth performance and elevated mortality secondary to respiratory disease.
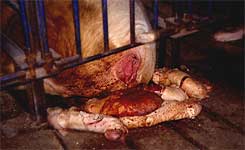 Third trimester abortion is a common observation following PRRSV infection of pregnant sows. |
 Premature parturition (early farrowing) following PRRSV infection results in the birth of weak born pigs, elevating pre-weaning mortality. |
 Mummies and stillbirths are common sequels to PRRSV infection during the third trimester. |
 A PRRSV-infected litter containing weak born piglets, mummies and stillbirths. |
However, the intensity of the disease appears to vary with isolate, and variation in the pathogenicity of PRRSV virulence has been observed in experimentally-infected animals. Studies found that pigs experimentally infected with 9 different U.S. isolates of PRRSV showed major differences in clinical disease, rectal temperatures, and gross lung and microscopic lung lesions. In these studies, animals infected with mildly virulent isolates or the LV showed transient pyrexia, dyspnea and tachypnea whereas infection with highly virulent isolates induced labored breathing, pyrexia, lethargy and anorexia. Furthermore, studies have reported that the impact on reproductive performance may be also isolate-dependent. Finally, the degree of clinical PRRS may be related to elevated viral concentration in blood and tissues, secondary to the ability of highly virulent isolates to replicate more efficiently in the host. Infection of susceptible pigs with highly virulent isolates of PRRSV resulted in longer periods of viremia, increased severity of clinical signs and mortality, and significantly higher viral loads in blood and tissues than those that were mildly virulent or cell-culture adapted. In the US, two new highly virulent isolates (MN-184 and 1-18-2) have been recovered from field cases. Besides their elevated pathogenicity, these isolates appear to have the ability to spread rapidly between herds, potentially via the airborne route.
Several other factors such as animal age and bacterial co-infection can influence virus replication and clinical signs. Studies comparing the effects of age determined that younger animals (4-8 weeks of age) infected with PRRSV demonstrated a longer viremia as well as higher excretion rates and replication rates in macrophages when compared to older (16-24 weeks of age) pigs. Additionally, it has been determined that certain bacterial agents such as Bordetella bronchiseptica and Mycoplasma hyopneumoniae appear to enhance the duration and severity of PRRSV-induced pneumonia and lung lesions. Furthermore, PRRSV infection has been reported to increase the susceptibility of pigs to Streptococcus suis type 2 infection, enhance the severity of Salmonella choleraesuis infection, and along with porcine circovirus type 2 co-infection, enhance the development of porcine circovirus associated disease (PCVAD). Therefore, swine veterinarians must conduct farm-specific diagnostic investigations to determine the role of PRRSV and the identity of the opportunistic agents present in the disease complex.



