Diagnostic Scenarios
1)Suspected acute outbreaks of A. pleuropneumoniae in a previously negative herd.
In this instance, the key factor is confirmation of whether or not the herd has broken down with APP. The best approach is to submit dead pigs or live affected pigs to the diagnostic laboratory for post-mortem examination. Samples of the typical acute pleuropneumonia lesions can be cultured using suitable techniques and media, and pure cultures subsequently serotyped. Depending on the availability of other tests, samples can be preserved for PCR, IFA and/or histopathology in case ancillary testing is needed.
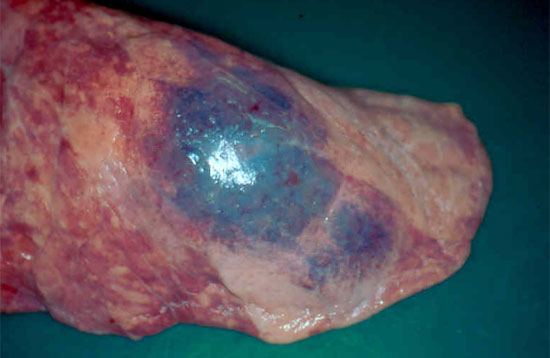
Figure 1. Pig lung showing an area of haemorrhagic pneumonia and early pleurisy associated with acute Actinobacillus pleuropneumoniae infection.
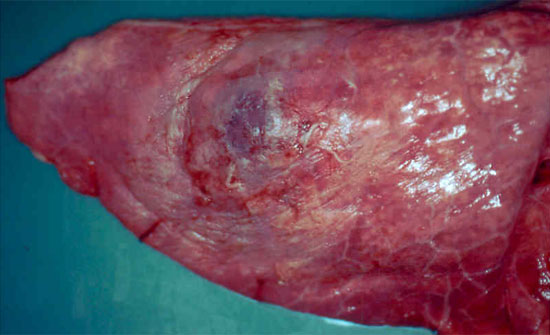
Figure 2. Pig lung showing a focus of subacute-to-chronic pneumonia with more extensive pleurisy, consistent with resolving Actinobacillus pleuropneumoniae infection.
In addition to post-mortem examination, swabs can be collected from the tonsils of live, affected pigs for ApxIV or serotype-specific PCR testing. Using a ‘nested’ PCR technique optimises the test sensitivity allowing swabs to be pooled in batches of 5 swabs per pool. In this way a representative number of pigs can be tested in a cost-effective manner.
The first set of blood samples for paired serology can be collected at this acute stage; ideally the pigs that are sampled should be tagged to enable the convalescent serum samples collected 3-4 weeks later to be matched correctly with acute samples on an individual pig basis.
2)Diagnosis of chronic A.pleuropneumoniae infection as part of ‘porcine respiratory disease complex’ (PRDC)
In complex pleuropneumonia situations where one or more co-infections could be involved, extending the investigation and sampling protocol to include material for other tests is advisable. This includes fresh lung tissue for bacteriology and virology and or PCR testing for other agents. Histopathology on 3 representative areas of pneumonic tissue provides further insight into the nature of the pneumonic lesions. In chronic cases of APP infection lesions become encapsulated, shrink and are eventually reduced to areas of chronic scarring. During the chronic stages, the number and viability of APP in lesions reduces dramatically and isolation rates are poor. In these instances, PCR testing of lung lesions and serology on recovered pigs in the batch provide more helpful diagnostic information. Antibodies are detectable from approximately 10 – 14 days post-infection.
3)Screening healthy pigs for freedom from A.pleuropneumoniae as part of a health assurance programme.
In addition to clinical inspection of herds and lung checks done on slaughtered pigs, representative numbers of pigs at high risk ages (i.e. growers or finishers) can be screened for APP by tonsil swabbing, as well as blood sampling for serology. PCR testing using ApxIV-based assays can also be used effectively in APP-free herds that have been vaccinated as an insurance against the possibility of severe disease, in the event of break-down in a naïve herd.
Difficulties that can be encountered with APP testing and interpreting results
Most difficulties arise when screening healthy pigs for freedom from APP, for the following reasons:
- Ideally herds should be negative for all serotypes of APP, however, non-virulent or low-virulent serotypes can be present in herds without any associated clinical disease.
- Most countries do not have agreed industry standards relating to which serotypes are acceptable to have in herds and which are not. As a start point, it is advisable that those with two Apx toxin genes (out of ApxI, ApxII and ApxIII) are deemed to be not acceptable. For pragmatic reasons, those with a single Apx toxin gene that are not associated with clinical disease could be allowed.
- The most widely-available screening tests (serology and PCR) are based on the ApxIV gene which encompasses all serotypes. Samples giving positive results require further exploration to determine their significance. This incurs further expense and delays in concluding the testing.
- Serotype-related serological tests are not widely available, and those that indicate virulence-related groups can have questionable specificity and need to be interpreted with caution.
- Serotype-specific PCR tests can be used where available, but these are generally confined to the major virulent serotypes. At best, negative results suggest that the specific virulent serotype is not involved, however, it might not be possible to determine which of the less important serotypes are present if they account for the ApxIV-positive results.
- Testing for virulence genes by PCR requires pure cultures of APP as these genes can occur in closely-related species such as Actinobacillus suis, that might also be present in the sample. When tonsil swabbing pigs for APP, the best approach is to take two swabs per pig, one plain swab for ApxIV PCR testing and one swab in Amies transport medium to use for APP culture in the event of a positive ApxIV PCR result.
- Culturing for APP from tonsil swabs is fraught with difficulties as cultures tend to get overgrown with the numerous commensal bacteria that colonise the tonsils unless more specialist techniques are used such as immune-magnetic separation or selective media that inhibit commensal organisms. If no APP isolate is obtained, the original DNA extract can be tested for ApxI, ApxII and ApxIII by PCR but results have to be interpreted with caution. Alternatively, pigs can be re-swabbed for another culture attempt or tonsils can be collected from culled pigs, for culture of deep tonsil tissue.
Despite the difficulties outlined, diagnostic testing for APP provides a useful contribution to the overall information that the practitioner has about the herd. The results of testing should always be interpreted in relation to the ‘bigger picture’ including clinical history, abattoir surveillance, post-mortem and culture results and serological screening.




