Introduction
Mycoplasma hyopneumoniae (M.hyo) is the causative agent of enzootic pneumonia in pigs and the most commonly isolated co-agent of the Porcine Respiratory Disease Complex (PRDC). Modern all-in, all-out (AIAO), multi-site production methods along with the development and availability of whole cell Mycoplasma bacterins have significantly reduced the impact of Mycoplasma pneumonia over past decades. Enzootic pneumonia is primarily a chronic respiratory disease of growing pigs and is manifested as a persistent non-productive cough with retarded growth rates, poor feed conversion, high morbidity and low mortality. However, after the emergence of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus and later Porcine Circovirus 2b (PCV-2b), PRDC and its primary agent M.hyo became economically and clinically a more important cause of growing pig mortalities. Field experience also indicates vaccines may have lost some of their protective advantages over the last couple of decades which could be indicative of evolving mycoplasma pathogenicity and/or the influence of the new and old co-pathogens which have likewise evolved.

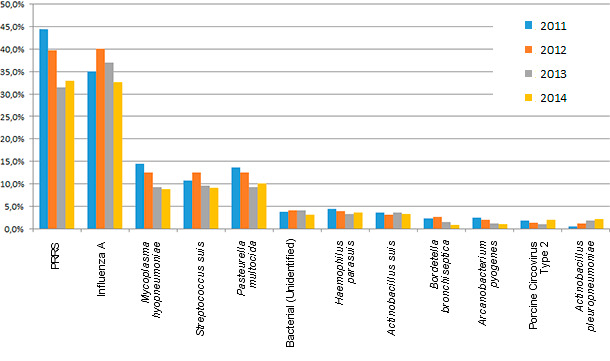
Figure 1: Diagnosis for etiologic agents as a percentage of submission. Source: ISU.
Vaccines
Interest in commercial vaccines started in the late 1960’s and by the late 1970’s a commercial vaccine was available. Since that time many M.hyo vaccine brands both monovalent and in various combinations have come into the marketplace on a global basis. These commercial vaccines dramatically reduced the clinical signs associated with enzootic pneumonia although the vaccines did not prevent infection, transmission, nor completely eliminate morbidity. Even so vaccine use is the accepted standard in the U.S. due to improvements in performance. Likewise antimicrobial studies have indicated that treatment will not prevent colonization or infection of the ciliated epithelial cells. It has also been demonstrated that vaccination of sows has little effect on colostrum anti-M.hyo antibody levels and further, maternally derived antibodies do not prevent colonization of piglets. Transmission studies indicate that mycoplasma infection rates are slow but the duration of infection compensates for the low transmission rates. Taken together this information brings into question the use of sow vaccination and high levels of sow herd antimicrobial use prior to or during M.hyo rollover elimination attempts.
Antimicrobials
Tetracyclines have long been utilized to control the effects of mycoplasma pneumonia and bacterial co-agents. M.hyo isolates are generally sensitive to lincomycin, kanamycin, erythromycin, valnemulin, tiamulin, enrofloxacin, oxytetracycline, tylosin, and sulfonamides. Those antimicrobials that are often successfully used in the U.S. include the lincosamides, pleuromutilins, fluoroquinolones, florfenicol, and aminoglycosides. Development of antimicrobial resistance has been demonstrated and must be considered when selecting a treatment antimicrobial. Fluoroquinolones are used to treat enzootic pneumonia in the EU. Both enrofloxacin and marbofloxacin have demonstrated significant field efficacy but decreases in susceptibilities has been documented after serial passages in pigs. In the field M.hyo resistance has not become a significant problem in individual or barn treatments except in those situations where PRRS virus, circovirus, and or influenza viruses are co-circulating. This situation frequently leads to treatment failure but not necessarily because of resistance.
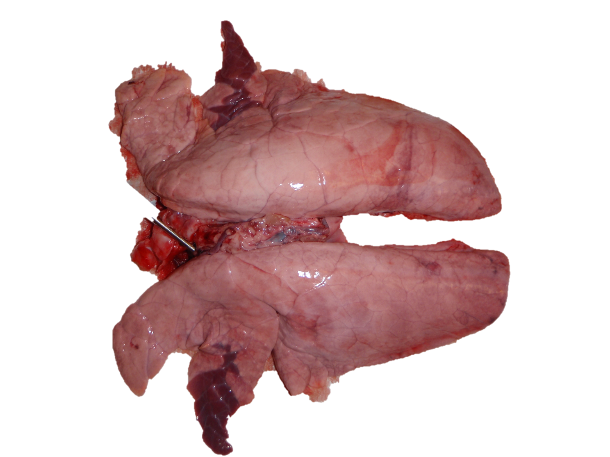
Figure 2: Lung experimentally infected with Mycoplasma hyopneumoniae
Diagnosis/Monitoring
Before the 1990’s there was little interest in purchasing M.hyo free pigs because of the cost of freedom, likelihood of re-breaks, and there was no apparent risk to already positive breeding herds by introducing positive replacements. Even so there are isolates of M.hyo which appear to be far more antigenic than most isolates thus a new introduction may not disrupt the health status of the breeding herd but it is highly likely this would destabilize the health of the growing pigs. Today there is significant interest in M.hyo freedom in commercial herds and systems. Over the past decade margins have continued to erode raising the awareness to elimination and exclusion of disease agents including M.hyo although it appears M.hyo can occasionally become an airborne agent it is unlikely that this is an efficient method of transmission except in high density growing pig locations. Likewise, the use of filtration has been promoted as an effective deterrent in the field.
Elimination
Interest in herd elimination of M.hyo began with the SPF certification program which had initially attempted to remove mycoplasma from all primary and secondary herd suppliers of breeding stock. After it became apparent that the biosecurity (bioexclusion) standards of that era were insufficient in preventing M.hyo introduction, secondary herds became exempt from this standard. As the SPF system fell into obscurity breeding stock companies began to consider freedom from the agent of enzootic pneumonia. Medicated early weaning batch farrowed piglets (MEW) and off-site group segregation post weaning was a successful elimination method when the breeding herd was asymptomatic. Today, nearly all genetic suppliers of replacement breeding stock in North America are free of M.hyo. These early successes at producing and maintaining M.hyo free herds and the restructuring of the industry to all-in, all-out (AIAO) multisite production has led to much greater commercial producer interest in freedom.

In the late 1990’s when PRRS “rollover” (herd closure to replacements followed by introduction of negative gilts) eliminations began in earnest, it was soon discovered that M.hyo could also be eliminated if negative gilt replacements were available after the closed period. The value of M.hyo freedom may range from $1.75 to $1.90/pig produced in the U.S. This value is in the ball park of comparisons made between before and after elimination following a herd closure and “rollover” with negative replacements. In commercial breed-to-wean farms which successfully attempt an M.hyo rollover elimination both whole herd vaccination and antimicrobials have been utilized expecting to reduce risk of failure during the herd closure period. Successful elimination has been accomplished without these treatments as discussed previously.
Summary
There remains a need for ongoing M.hyo research especially field studies on elimination procedures, bioexclusion methodologies and new vaccines which inhibit respiratory tract colonization. It has been this author’s experience that M.hyo elimination is less costly and simpler than PRRS elimination, is not as likely to be an airborne agent, and far less likely to be spread by fomites. Thus it could be a good candidate for regional or national eradication efforts.