Swine influenza vaccines resemble the flu vaccines for humans, but not entirely
The current commercial swine influenza virus (SwIV) vaccines are mostly traditional inactivated (or killed) vaccines for intramuscular (IM) injection. Such vaccines protect by inducing antibodies against the major viral surface protein, the hemagglutinin (HA). The weakness of these neutralizing antibodies is that they target the most variable parts of the HA. It is for this reason that the strains in the human flu vaccines are updated every few years, in order to match the circulating strains (Dormitzer et al, 2011).

SwIV vaccines share similarities with the killed vaccines for humans, but there are essential differences too. While the human vaccines generally contain purified viral surface antigens without adjuvant, most SwIV vaccines are whole virus preparations with an oil-based adjuvant. And unlike for the human vaccines, antigenic dose and vaccine strains are not standardized for SwIV vaccines (Van Reeth and Ma, 2013). In keeping with the antigenic and genetic differences between SwIV in Europe and in North America, the vaccines for each geographical region are produced locally and contain entirely different strains. The most widely used vaccine on the European market is trivalent and contains avian-like H1N1, human-like H1N2 and H3N2 SwIV from around 2000 in combination with a carbomer adjuvant. An oil-adjuvanted bivalent vaccine is also frequently used. It is based on an avian-like swine H1N1 and a human H3N2 strain isolated more than 35 years ago. Similar vaccines in the US contain representative strains of up to 3 different H1 SwIV lineages, and one or 2 H3N2 lineages. A monovalent vaccine based on the 2009 pandemic H1N1 virus was recently launched in both continents.
The complexities of SwIV vaccine strain selection
SwIV have undergone more changes than ever during the last decade, and the lack of SwIV vaccine strain changes and recommendations has become an important issue. Though virtually all SwIVs have derived their HA from viruses that once circulated in the human population, the epidemiology of influenza is much more complex in swine than in humans (Lewis et al, 2016). Multiple introductions of influenza viruses from humans to swine in different countries and the fact that pigs don’t travel like humans do, have contributed to an enormous genetic and antigenic diversity of SwIVs. This is illustrated in Fig. 1 for the H3N2 subtype.
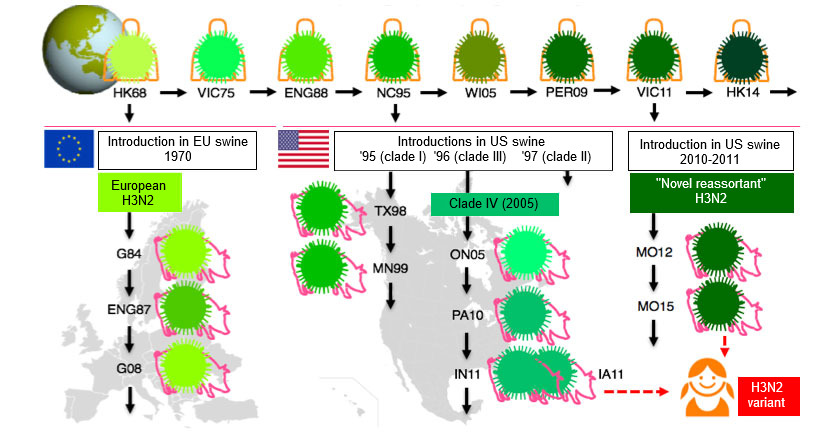
The H1N1 and H1N2 subtypes of SwIV are even more diverse. Three H1 lineages are circulating simultaneously in many European countries: avian-like, human-like and the 2009 pandemic H1. The amino acid sequence of their HAs differs by as much as 20-25%, and there is minimal serologic cross-reactivity between them. Moreover, the prevailing lineages and clades (sub-classification within a lineage) differ in different European regions, and still other clades are circulating in North America and Asia.
It is important to stress that, unlike the human flu vaccines, many of the commercial SwIV vaccines show a relatively broad protection against drift variants within a given H1 or H3 lineage. This can be explained by the presence of adjuvants in these vaccines, which increase the height and cross-reactivity of the antibody response. The challenge, however, is how to protect pigs against multiple co-circulating H1 and H3 viruses from various lineages and clades. This would require multivalent vaccines containing at least 5 different H1 and H3 SwIV strains. The appropriate strains would not only differ in different continents and regions, but would also need regular updates. Such polyvalent SwIV vaccines are both technically and economically elusive. The development of vaccines that can offer a broader, cross-lineage and clade protection would be a far more preferable approach.
Heterologous prime-boost vaccination may offer a broader protection than a matched polyvalent vaccine
Influenza researchers are trying to stimulate antibody responses towards parts of the HA that are the least variable from strain to strain. The epitopes in such parts are largely ignored by traditional vaccines and vaccination strategies because they are “subdominant”. One approach that seems promising is choosing two antigenically very different strains within the H1 or H3 subtype for the primary and subsequent booster vaccinations. Because such strains show little overlap in the most variable, immunodominant epitopes, antibody producing cells will be redirected to conserved, subdominant epitopes. Our research group at Ghent University has demonstrated the potential of this “heterologous prime-boost” strategy in a pig study with experimental inactivated H3N2 SwIV vaccines. Influenza naïve pigs were first injected with a European H3N2 strain followed 4 weeks later by a North American H3N2 strain, or vice versa, as shown in Fig. 2.
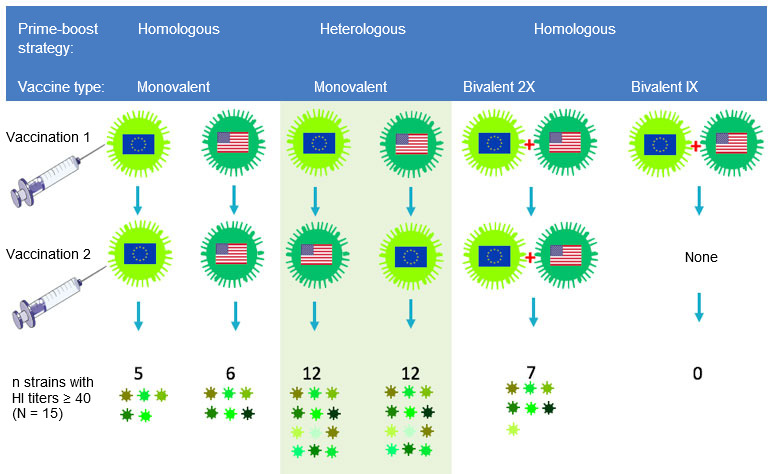
The resulting antibodies cross-reacted with about 80% of a panel of 15 different H3N2 virus strains of both human and swine origin, as compared to less than 40% in the homologous prime-boost control groups (Van Reeth et al, 2017). Antibodies against both vaccine strains were found only after heterologous prime-boost vaccination, or after 2 injections of a bivalent vaccine combining both strains, but the extent of cross-reactivity was much higher in the former groups. Using different vaccines for the first and booster vaccination does not only appear to be more immunogenic than any of the existing SwIV vaccines, it could also reduce the total amount of vaccine needed.
Importantly, not just any combination of antigenically distinct H3N2 or H1N1 SwIV strains will work well. Also, the order of administration of vaccine strains appears to have a dramatic effect on the antibody response. We are currently trying to deduce the scientific basis for these observations and to further improve prime-boost regimens with H3N2 as well as H1N1 and H1N2 viruses.

From an experimental setting to the field
Unfortunately, the effects of priming and boosting with different commercial SwIV vaccines have not been studied so far and we cannot yet recommend specific prime-boost regimens for use in the field. This alternative approach would require a liberal attitude from vaccinators. It is also unlikely that a single prime-boost regimen would be appropriate for animals of all ages. Yet we do believe that a few selected regimens may alleviate some of the biggest hindrances to effective SwIV vaccination. Vaccination of young piglets with strains that differ from the field strains and those used to vaccinate sows could, to some extent, circumvent the inhibiting effect of maternal antibodies. In addition, repeated sow vaccinations with alternate strains are likely to be more immunogenic than using the same strains time and again. Ideally, pre-existing active immunity should also be considered in the design of vaccination strategies. Indeed, gilts and sows have usually been previously infected with one or more SwIV strains. This pre-existing immunity has been shown to enhance and, in many cases, broaden the antibody response to killed vaccine, especially if the vaccine strains differ from the ones causing past infections.
The policy of regularly updating vaccine strains is not only impractical for swine influenza vaccines, it also has limitations. We should not forget that the success of vaccination will depend on many factors other than the vaccine strain match, such as the influenza infection history, the adjuvant and antigenic dose in the vaccine (Van Reeth and Ma, 2013). These other factors are likely more important in swine than in the human situation.
Acknowledgements
Influenza research in the author’s lab is supported by the Special Research Fund of Ghent University, the Belgian Federal Public Service for Health, Food Chain Safety and Environment, and the European Commission.



