Farm description
The farm was located in Wielkopolska region of Poland and consisted of 250 sows. The farm has produced breeding animals therefore all gilts classified as prospective mothers were kept until they reached 100 kg body weight. Weaners and the gilts without prospect were sold to fattening farm after weaning. Insemination was used in reproduction. Technological groups consisting of 10 pregnant sows were formed and their piglets were weaned at 28 days of age. Approximately 6200 weaners were reared, annually. The “all in – all out” procedure was maintained. The environmental conditions in which the animals were reared were good. The health status of the farm was identified as very high. The herd was negative for Brachyspira hyodysenteriae, Salmonella spp., and Pasteurella multocida causing PAR, PRRSV, Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae, Leptospira spp., and positive only for Bordetella bronchiseptica and Lawsonia intracellularis. The cases of PMWS were not observed either. In breeding herd routine prophylactic vaccination against colibacteriosis (gilts at 8 and 3 weeks, and sows at 3 weeks before delivery) and against erysipelas and parvovirosis (gilts - at 5.5 and 6.5 months of age and sows at 3 weeks before delivery) were carried out in the farm. There have been no vaccination against PCV2.

Case description
During the first visit, a sudden death of animals, with no prior symptoms of disease, and clinical symptoms such as massive facial, ears and forechest oedema (Fig. 1), as well as an increase of body temperature to 41.5ºC, were observed. In individual cases, neurologic symptoms (opistothonus, lateral position, rowing movements of limbs) were also noticed. Initially, the symptoms were observed in a group of gilts of 70-100 kg body weight. Morbidity rate increased up to 15% and mortality rate up to 6%. Over the next month, similar symptoms occurred but with slightly lower intensity, and they included units with younger animals – weaners and gilts up to 70 kg of body weight. Over the next weeks, similar symptoms (with the exception of neurological symptoms) were observed in groups of weaned sows and in the farrowing units and suckling piglets.
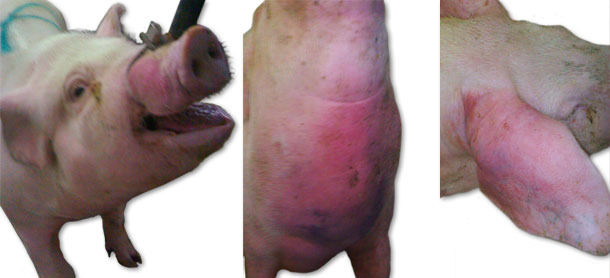
Fig. 1. Oedema symptoms
Necropsy and laboratory tests
Four sick animals were submitted for examination to the diagnostic laboratory. They exhibited clinical symptoms such as massive pitting oedema of lower body parts, subcutaneous accumulation of fluid in the sternum area in the case of two weaned pigs (Fig. 3) and body temperature of 41.0 to 41.3ºC. Necropsy showed subcutaneous gelatinous infiltrations, especially in the area of the lower body parts (Fig. 2, 3) and serofibrinous pericarditis (Fig. 4) and pleuritis.
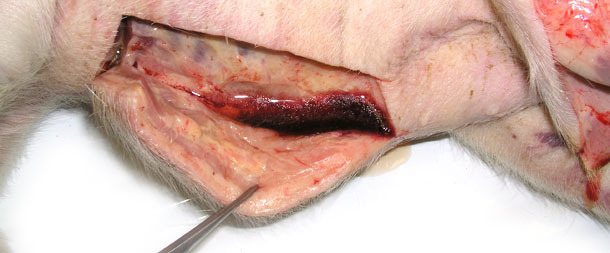
Fig. 2. Swelling of the subcutaneous tissue, a large amount of fluid egressing after incision of skin in the lower part of the body.
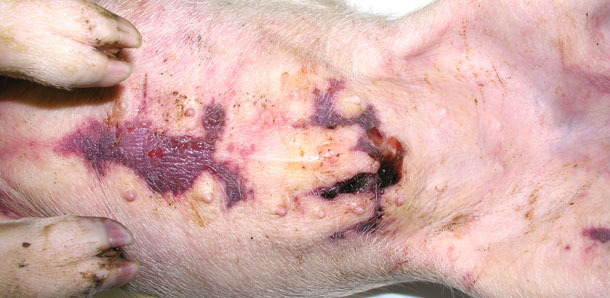
Fig. 3. Accumulation of fluid beneath the skin in the sternum area.
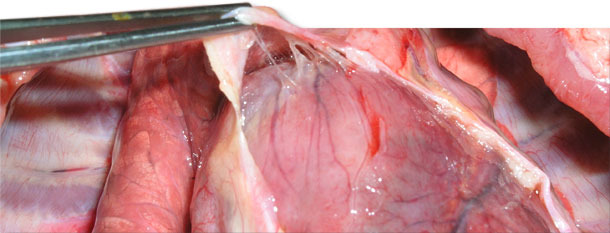
Fig. 4. Delicate and fragile fibrin between the epicardium and pericardial sac.

Bacteriological examination was performed following the pathologic assessment. Since there were no lameness or nervous symptoms in the case of tested animals, no material was taken from the joints and the cerebrospinal fluid. The material tested was collected aseptically and the swabs were taken shortly after opening the body cavities.
The material was seeded on horse agar with nurse culture of Staphylococcus epidermidis and McConkey agar. The identification was performed with the use of biochemical tests (Vitec) or by PCR.
Lab results
Bacteriological studies showed growth of only Haemophilus parasuis (H. parasuis). The colonies were very small (like pin pricks), nonhemolytic, showing satelitism (increase in colony size) around the source of NAD - a strain of Staphylococcus epidermidis.
In the case of swabs derived from pathological changes (weaned pigs), the material was also analysed with the use of PCR method directly. The results are shown in Table I below.
Table I. Haemophilus parasuis laboratory diagnosis on serosal surfaces and serum.
| tissue | weaner 1 | weaner 2 | weaner 3 | weaner 4 |
| Isolation + PCR/tissue material PCR | ||||
| pleura | +/+ | +/+ | +/+ | +/+ |
| pericardium | +/+ | +/+ | +/+ | +/+ |
| peritoneum | -/- | +/+ | -/- | -/- |
| serum | +/+ | -/- | -/- | -/+ |
The presence of H. parasuis in the lesions typical for Glässer disease was confirmed. The bacteria or the bacterial DNA (PCR) was also identified in serum, which showed that the two of four examined pigs had bacteraemia.
Furthermore, after detection of H. parasuis in four necropsied weaned pigs, 20 nasal swabs were taken from the weaners, gilts and sows in order to compare characteristics of the strains from sick and healthy (carriers) animals. Nasal swabs were analysed with the use of isolation and identification of microorganisms (biochemical identification and final strain confirmation with the use of PCR method).
Ten H. parasuis isolates from the herd – four from examined weaned pigs and six other isolates obtained from healthy animals, were analysed using ERIC PCR molecular typing method (Fig. 5). All isolates obtained from the diseased animals had identical ERIC PCR profiles. Interestingly, identical strains were also found in nasal samples of two out of six healthy animals. The remaining four animals had different strains of H. parasuis in nasal cavities that were characterised by different ERIC PCR profiles.
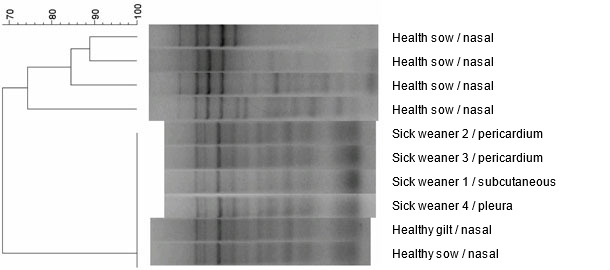
Fig. 5. Dendrogram created by comparison H. parasuis strains, obtained from the farm, by the use of ERIC PCR method.
Therapy
The treatment procedure consisted of introducing amoxicillin and clavulanic acid i.m. in the case of the first animals indicating the clinical symptoms, and implementing metaphylactic treatment with amoxicillin (water treatment, 14 days, 20 mg/kg b.w.) in the case of the remaining animals. Such a treatment has provided a permanent therapeutic result.
Conclusions
In high health status herds, the H. parasuis infection often has acute form. Animals of all age groups are susceptible to the infection. Symptoms such as fever, apathy, respiratory disorders, pain observed after pressing on the abdominal muscles (muscular defence), swollen joints and lameness are characteristic for this form of Glässer disease. Nervous symptoms, as well as subcutaneous oedema, similarly like in the described case have been also noted.
Pathological changes occurring in the described case of the acute disease are characteristic for the acute serofibrinous inflammation. Such changes are not pathognomonic and therefore the diagnosis must be based on the laboratory analysis.
A specific location of the oedema (lower parts of the body) and its pitting character may suggest that it results from a heart failure (cardiac oedema), particularly congestive heart failure and consequent blood stagnation in the systemic circulation. This statement is contradictory with confirmed presence of H. parasuis bacteria in the subcutaneous tissue in this particular case. This may suggest swelling inflammation caused directly by bacterial agent. However, this fact can be explained by bacteremia, which is the detection of the presence of bacteria in the subcutaneous tissue, derived from damaged blood vessels, in this particular case.
The ability to differentiate strains of H. parasuis that circulate herd can be important as the epidemiology of H. parasuis is complex and the infection caused by this microorganism is a dynamic process. For this purpose, H. parasuis is often subjected to different typing techniques, which allows strain identification within species (ERIC PCR). Especially, in herds with a high health status there is a dominance of certain strains or strain that can cause disease. Hence, the ability to differentiate strains of H. parasuis at the level of the herd is important. In the described case it has been demonstrated that six isolates had identical ERIC PCR profile and belonged to one pathogenic strain of H. parasuis. Its presence was also confirmed in clinically healthy animals. The above statement and permanent therapeutic effect of the applied antibiotic therapy (in the absence of vaccination), may indicate growing immunity in the herd, against clinical outcome of the infection with the previously pathogenic strain.