The shedding of PRRS virus in an infected animal occurs through nasal secretions, saliva, urine, feces, blood, milk, and semen, and the duration of the excretion period varies depending on the type of secretion, the animal's age, its immune status, and the specific strain of PRRS. The virus can persist for long periods of time in lymphoid tissue (up to 251 days post-infection in tonsils has been reported), even months after the clearance of viremia.
In a PRRS control program, it is essential to select sample types and sampling techniques that allow carrier animals to be detected in the final phase of excretion, with lymphoid tissue and saliva being the samples of choice.

The development of oral fluid sampling techniques represented a significant advance in increasing detection capacity, especially in situations of low prevalence and in the final stages of infection. However, the presence of inhibitors in this type of sample requires optimal sample preservation prior to RT q-PCR. Furthermore, CTs are often high, which limits the ability to obtain the viral sequence.
The tonsil scraping method described a few years ago had a significant limitation: it required abrasion of the tonsil to obtain tonsil exudate, which was performed with instruments such as spoons, making it difficult to implement in practice on live animals. In 2024, Peng Li (University of Iowa) published an adaptation of the scraping method to a less invasive technique called tonsil scrubbing, which showed a greater ability to detect the virus compared to sera from infected sows.
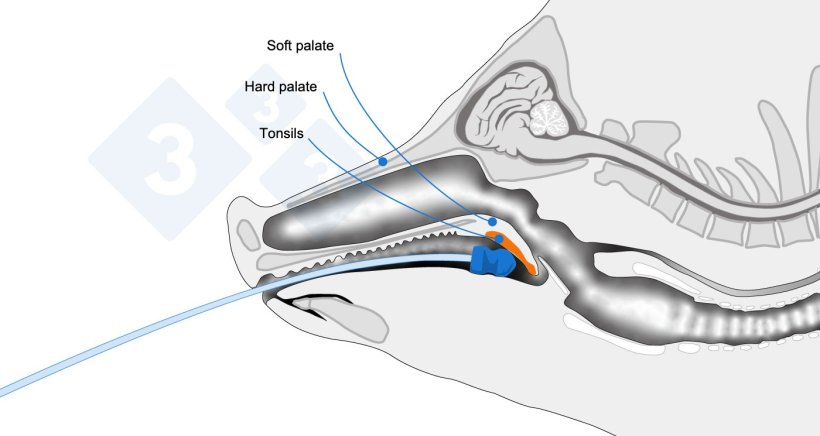
Anatomical diagram indicating the sampling area.
This method was subsequently evaluated on several farms in the Aragon and Catalonia regions of Spain, following the protocol described by Peng Li (2024), with some modifications. Insemination catheters with sponge tips were also used, as described by Peng Li, but the following variations were introduced:
- No gauze was attached to the tip of the catheter.
- The animal was restrained during sampling to facilitate direct sampling of the tonsil, which would be difficult in moving animals.
- The samples were collected in Falcon tubes for processing.
The study was conducted on two types of farms:
- Finishing farms with piglets that were naturally infected during the nursery phase.
- Gilt development units, previously infected naturally during the nursery phase, with the aim of detecting chronic carriers and preventing the introduction of viruses when transferring them to commercial farms.
On the first farm, the pen was considered the epidemiological unit. Five animals were selected from each pen for simultaneous blood sampling and tonsil scrubbing from the five animals, in addition to a group oral fluid sample.
The objective of the study was to estimate the detection capacity (absence or presence) at the group level with pools of blood, tonsil scrubbings, and oral fluids.
On the other two farms, individual samples of serum, tracheobronchial scrapings, and tonsil scrubbings were taken, and the analytical results were compared by animal.

Collection of tonsil scrubbing samples.
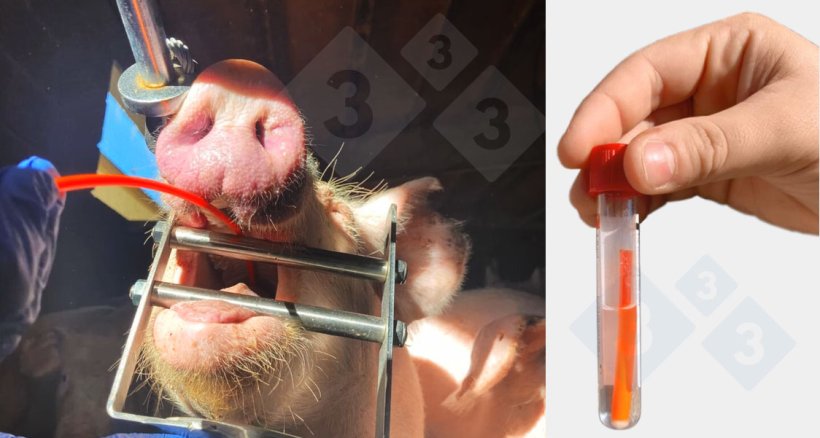
Collection of tracheobronchial scraping samples.
All samples were analyzed using RT q-PCR, with cT values < 40 considered positive. The degree of positivity (viral load) of each type of sample was also compared.
The results showed that tonsil scrubbings detected a higher proportion of PRRS virus-positive groups (100%) than oro-tonsillar scrapings obtained at 140 days of age and compared to the results obtained with serum and oral fluids (66.6%). In addition, positive tonsil scrubbings had a lower average cT (30.7) compared to serum (35.6) and oral fluids (35.5).
PRRSV detection rate
| Sample type | 10 weeks | 20 weeks |
|---|---|---|
| Blood | 100% | 65.51% |
| Oral fluid | 100% | 65.51% |
| Tonsil scrubbing | 100% |
Average cT value of PRRSV
| Sample type | 10 weeks | 20 weeks |
|---|---|---|
| Serum | 23.16 | 35.67 |
| Oral fluid | 27.00 | 37.00 |
| Tonsil scrubbing | 30.67 |
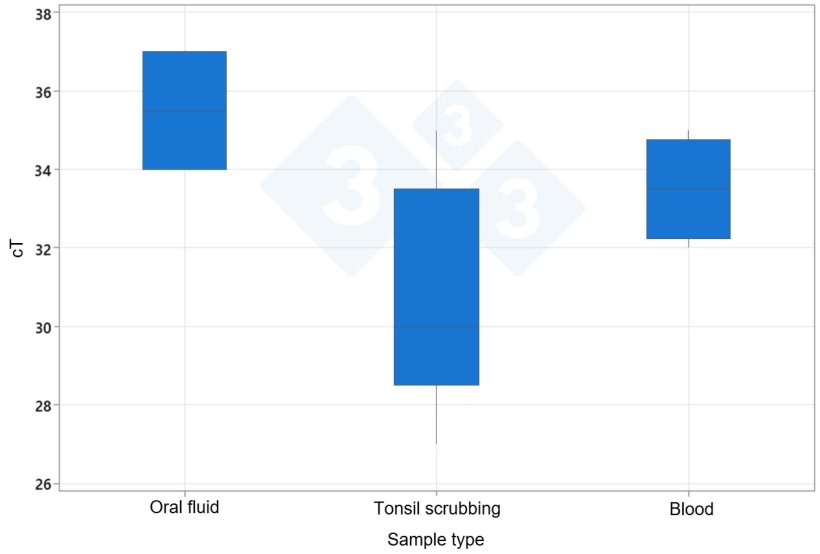
Average cT value of PRRSV according to sample type
In the study conducted with chronically infected individual animals, detection rates were higher in tonsil scrubbings (61.5%) compared to serum samples (7.7%) and tracheobronchial scrapings (30.8%). The mean viral load of positive samples was higher in tonsil scrubbings and tracheobronchial washes (cT 34.1 and 33.0, respectively) than in the few positive serum samples (cT 36.0).
| Sample | Negative | Positive | Mean cT |
|---|---|---|---|
| Tracheobronchial scraping | 50 (33.56%) | 4 (30.77%) | 33.50 |
| Tonsil scrubbing | 46 (30.87%) | 8 (61.54%) | 34.13 |
| Serum | 53 (35.57%) | 1 (7.69%) | 36.00 |
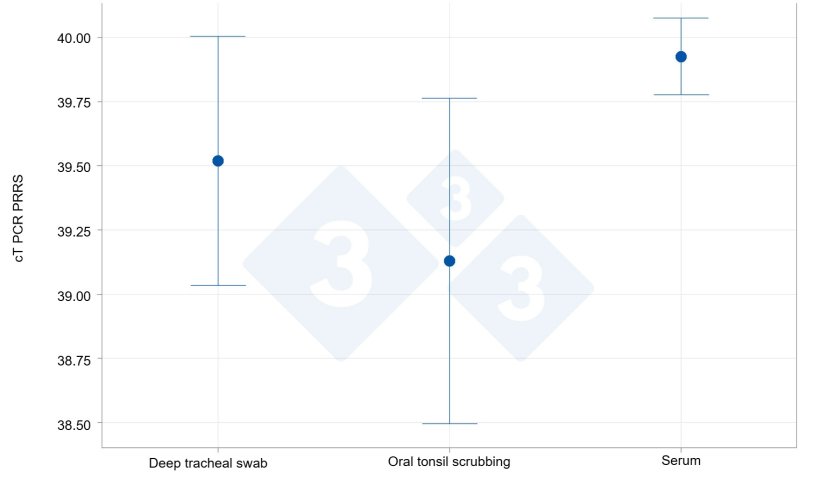
Detection capacity in tonsil scrubbings compared to sera and tracheobronchial scrapings
This new sampling technique could provide a useful tool for better understanding the dynamics of infection in sows infected with virulent strains, given the prolonged viral excretion that can be observed, especially around the time of farrowing, and their potential role in transmitting the virus to their progeny.
In the near future, its usefulness in other scenarios, such as gilt acclimation, the elimination of carrier animals in eradication programs, as well as its ability to detect and monitor other pathogens such as Mycoplasma hyopneumoniae or the swine influenza virus, will be assessed.




