When the clinical signs on the farm and the lesions observed in the piglets lead us to suspect that there is a Haemophilus parasuis infection (see chapter on “Clinical and pathologic diagnosis”) we have to confirm this diagnosis in the laboratory.
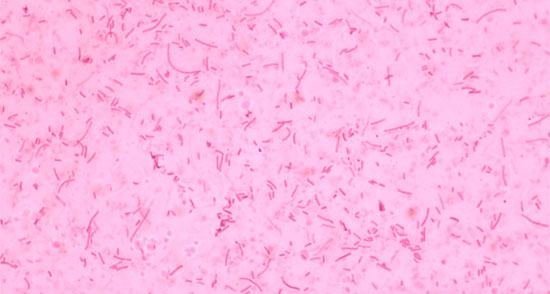
Fig. 1 Gram stain of Haemophilus parasuis. Short gram negative bacilli are observed and others that are longer (pleomorphic)
Sample taking
Since Glässer found the association between the bacillus and the polyserositis lesions in piglets 32 years passed before Hjärre and Wramby achieved its isolation in the laboratory in 1943. Since then, the isolation of Haemophilus parasuis from the characteristic lesions has been the conclusive technique or “gold standard” for the diagnosis of the disease. However, H. parasuis is a labile bacteria with complex requirements to grow in the laboratory and its isolation in pure culture can be difficult. To increase the possibilities of achieving isolation of this bacteria it is essential to have the right samples, taken while avoiding contamination and adequately transported.
The samples should be taken from animals that present acute signs of the disease, mainly respiratory distress or arthritis, but also from animals with nervous signs. If possible, animals should be chosen that have not been treated with antibiotics. The animals affected chronically are not suitable, since the bacterium cannot be isolated from their chronic lesions (fibrous polyserositis). The samples should be taken from systematic lesions (pericarditis, arthritis, peritonitis,…) with the help of swabs or by collecting samples from the liquids accumulated in distinct cavities. In animals with nervous symptoms a sample of cerebrospinal fluid can also be taken. Samples from the lung are not suitable for the diagnosis of Glässer’s disease and they should be used only when the animals present pneumonia. This is because H. parasuis is a coloniser of the upper respiratory tract of piglets and the presence of the bacterium in the lung may be due to a post mortem invasion from the nose. In this way a strain of H. parasuis would be isolated that would not really be implicated with the disease. For the same motive nasal or tonsillar samples cannot be used in the diagnosis. To increase the survival possibilities of the bacterium, the samples should be transported to the laboratory as soon as possible (best before 2 days) and in Amies medium under refrigeration.
Laboratory techniques of diagnosis
H. parasuis is a gram-negative bacillus of the Pasteurellaceae family that requires NAD to grow. Therefore, in the laboratory it grows in agar chocolate or in blood agar with a striation of Staphylococcus which proportions the NAD. H. parasuis grows slowly and requires 1-3 days to produce small colonies. The identification of H. parasuis must include the differentiation of similar bacteria such as Actinobacillus minor, Actinobacillus indolicus and Actinobacillus porcinus. This differentiation is important because these Actinobacilli can also be isolated from the respiratory tract of pigs, and although they are considered to be non-pathogenic or low pathogenic, they have been isolated in pure culture of pneumonic lungs, and even from systemic lesions in the case of A. porcinus. The differentiation can be done through biochemical tests, specifically indole production, urease activity and catalase activity.

Fig. 2 Growth of Haemophilus parasuis in pure cultivation on a plate of agar chocolate.
Table 1. Differentiation of Haemophilus parasuis and Actinobacilus minor, A. indolicus and A. Porcinus using biochemical tests
| H. parasuis | A. minor | A. indolicus | A. porcinus | |
| Catalase | + | - | + | - |
| Indole | - | - | + | - |
| Urease | - | + | - | - |
The introduction of PCR in the diagnosis of infectious diseases offers an advantage in the diagnosis of microorganisms that are difficult to cultivate or that have a very slow growth. In the case of H. parasuis, the most used PCR is that published by Oliveira et al in 2001. This technique can substitute the biochemical identification of the bacterial isolations or be used 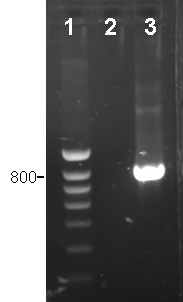 directly in clinical samples. In this last case, this PCR has a sensibility of 102 CFU/mL. As in the case of the isolation, the nasal or tonsilar samples are not suitable for the diagnosis. In the cases in which the isolation cannot be done, for example when there are an excessive number of samples, this specific PCR may help in the diagnosis. However, the preparation of the bacterial isolation allows us to carry out additional tests, such as antimicrobial susceptibility or typing, which help in the control of the disease.
directly in clinical samples. In this last case, this PCR has a sensibility of 102 CFU/mL. As in the case of the isolation, the nasal or tonsilar samples are not suitable for the diagnosis. In the cases in which the isolation cannot be done, for example when there are an excessive number of samples, this specific PCR may help in the diagnosis. However, the preparation of the bacterial isolation allows us to carry out additional tests, such as antimicrobial susceptibility or typing, which help in the control of the disease.
Specific PCR for detection of Haemophilus parasuis.

1: marker of molecular weight
2: negative sample, H.parasuis not detected
3. positive sample, lesion produced by H. parasuis


