PCV2 is a ubiquitous virus in pigs and wild boars. However, this does not necessarily correlate with the distribution of porcine circovirus at a worldwide level. It is true that the disease has been identified in every continent, but there are still countries in which the infection is present, but where the clinical process has not yet been identified (lack of diagnostic methods or a real absence of the disease?). This subject would again have a connection with the existence of distinct genotypes of PCV2 with an apparently distinct pathogenicity. Could it be that some countries have pigs with a low pathogenic genotype compared to other countries? A recently published Danish study suggests the existence of a third genotype of PCV2 (genotype 3) that was detected in pig samples corresponding to the years 1980, 1987 and 1990. The existence of this third genotype could indicate that a “genotypic gradient” has been produced since the 1980s until now which would follow the order of genotypes 3, 2 and 1, the latter apparently being related with the epizootic outbreaks of the disease which occurred in Denmark. We do not currently know if this is extrapolatable to the rest of Europe, and if this “genotypic gradient” has also occurred or not in other regions of the world.
Apart from the domestic pig and the wild boar, it is unknown if any other species are susceptible to the PCV2 infection. At least there has not been any PCV1 or PCV2 infection found in any of the following studied species: dog, cat, goat, horse, bovine, rabbit, rat, mouse, chicken or human (including humans with a theoretical high risk of infection such as veterinarians).
The dynamics of the PCV2 infection have been studied on farms with and without the PMWS by using sero-profiles and also by what we call PCR-profiles (detection of the agent genome through PCR in stratified samples according to age, as well as the sero-profiles). Currently we could also talk about profiles of virus load (Fig. 1), based on the use of quantitative PCR.
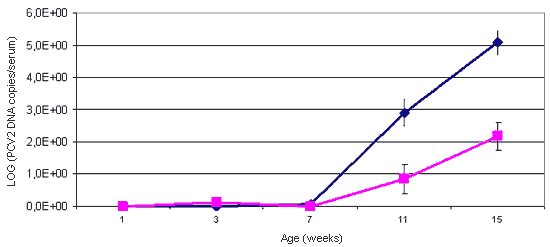
Figure 1. The use of quantitative PCR for the detection of PCV2 allows the viral load of the studied animals to be established. The animals affected with PMWS (blue line) present a viral load in serum which is significantly higher than that of healthy animals (pink line).
The dynamics of antibodies against PCV2 are relatively homogenous and predictable on the majority of farms (Fig. 2). The maternal humoral immunity would have a variable duration between 4 and 12 weeks old, with the sero-conversion being observed between 8 and 16 weeks old. On the contrary, the dynamics of PCV2 detection (using PCR techniques) can be relatively erratic, with infections in very young pigs (even in the farrowing room, Fig. 2A) and detection of the virus in practically every age group, or with the peak of infection in one age group and the detection of PCV2 in a sporadic manner in other groups of animals (Fig. 2B). Between these two extremes, practically any intermediate situation can be found. However, it is true to say that there is not a high percentage of pigs infected with PCV2 found at the earliest ages, and that this peak is usually found with pigs between 6 and 14 weeks old. In this context, it should be pointed out that piglets from sows with a low antibody resistance to PCV2 or with a positive result to the detection of the virus (by PCR) around the time of farrowing, have a higher risk of suffering PMWS.
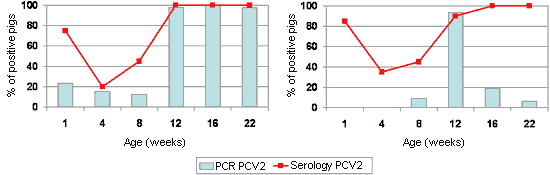
Figure 2. Sero- and PCR-profiles in front of PCV2 on two farms affected with PMWS betwwen 9 and 14 weeks old (inset with dotted line). Left: Farm in which we can see a certain percentage of piglets in the first week of life with the infection (measurement by standard PCR) as well as high percentages of affected pigs of different ages. Right: Farm in which there was a peak of PCV2 infection around the time that the disease appeared.
It is important to mention, however, that the dynamics of the PCV2 infection can be very similar on farms with or without disease (Fig. 3), which reinforces the multifactorial character of the infection, and also the serologic techniques and even the detection of PCV2 are not sufficient to diagnose the disease.
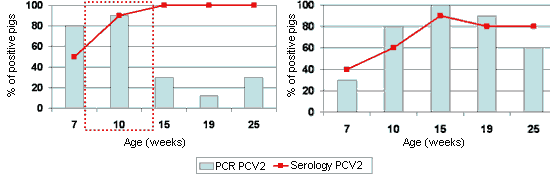
Figure 3. Sero- and PCR-profiles (from 7 weeks old) in front of PCV2 on one farm affected with PMWS between 8 and 14 weeks old (inset with dotted line) (Left), and another apparently normal farm with no symptoms of the disease (Right).
On the other hand, PCV2 has been detected (by PCR) in pig serum at least in pigs under 28 weeks old, and in some cases this positivity (without knowing if it is continuous or intermittent) has lasted at least up to 21 weeks. It is for this reason that it is believed that PCV2 can establish a persistent infection in a certain percentage of pigs.
The virus has been detected in distinct potential excretion tracts, including nasal secretions, saliva, tonsils, tracheal mucus, urine, feces, semen and ocular secretions, which would indicate that PCV2 could potentially be excreted through any of the organic tracts. Moreover, the animals suffering from PMWS not only present a significantly higher viral load in serum, they also excrete a significantly higher quantity of the virus.
It is assumed that the most probable route of PCV2 transmission is the oronasal tract, which would indicate that horizontal transmission (sow-piglet or piglet-piglet) is a frequent or very frequent occurence. If practically all the animals on a farm are infected between 2 and 4 months old, PCV2 must be a highly contagious virus. However, perhaps the most recent important fact is the demonstration that PCV2 is not only transmissible, but it can also transmit porcine circovirus from a sick pig to a healthy one. This may seem very obvious to the majority of readers, but it wasn’t from a scientific point of view. It is also true that in some of documented transmission models, there is nearly always the co-infection of some other pathogen. This should not surprise seeing as this is what usually happens under field conditions! It is also known that PCV2 can be detected in semen; however, it remains unknown whether or not this is sufficient to cause an intrauterine infection with pathological consequences (embryonic death, fetal death, or the subsequent development of porcine circovirus in the infected fetuses).



