Evaluation of Sample Materials for the Detection of Mycoplasma Suis in Swine
Background and Objective
Mycoplasma suis (M. suis) is a hemotrophic bacterium that adheres to porcine erythrocytes and can cause infectious anemia, particularly in young pigs or immunocompromised animals. Although many infections remain subclinical, clinical manifestations such as fever, icterus, cyanosis at the extremities, lethargy and reduced performance may occur in the presence of stress or co-infections (Felder et al. 2011). Reliable and early diagnosis is essential for effective herd management.
EDTA blood is widely regarded as one of the most reliable sample types for PCR-based detection of M. suis, although alternative sample types may offer practical advantages in field settings. However, data regarding the diagnostic performance of alternative matrices is limited. The aim of this investigation was therefore to evaluate and compare the diagnostic suitability of various sample types for M. suis detection by real-time PCR, with the goal of identifying reliable and potentially more flexible alternatives to EDTA blood.
Material and Methods
A total of 21 PCR-confirmed M. suis-positive sows from a positive herd were selected for sample collection. Four different sample types were collected from each animal: EDTA blood, serum, oral fluids and used needles from routine injections. In total, 84 samples (21 of each type) were submitted to SAN Group Biotech Germany GmbH for analysis. The samples were tested using real-time PCR targeting M. suis-specific gene sequences (INgene® q Mycoplasma suis). The evaluation focused on the proportion of positive samples for each material type and the corresponding Ct values, which served as indicators of both diagnostic sensitivity and relative pathogen load.
Results
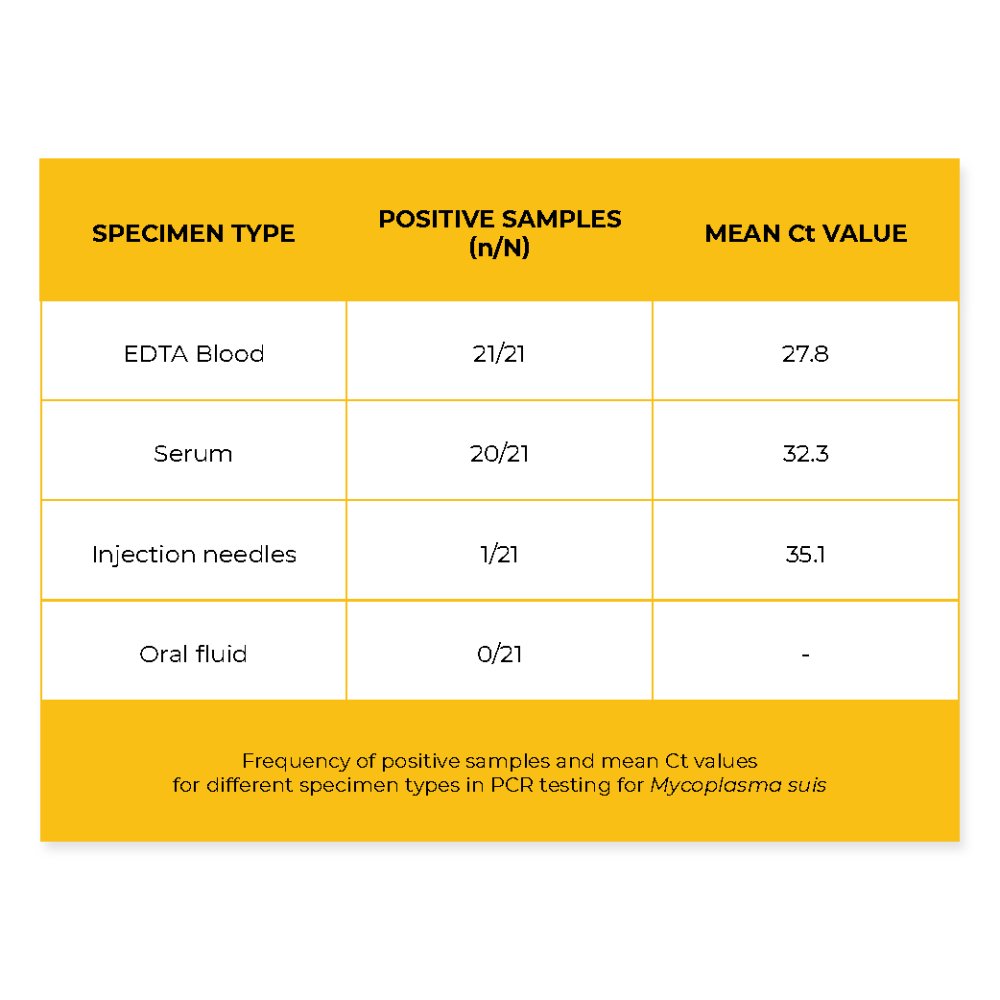
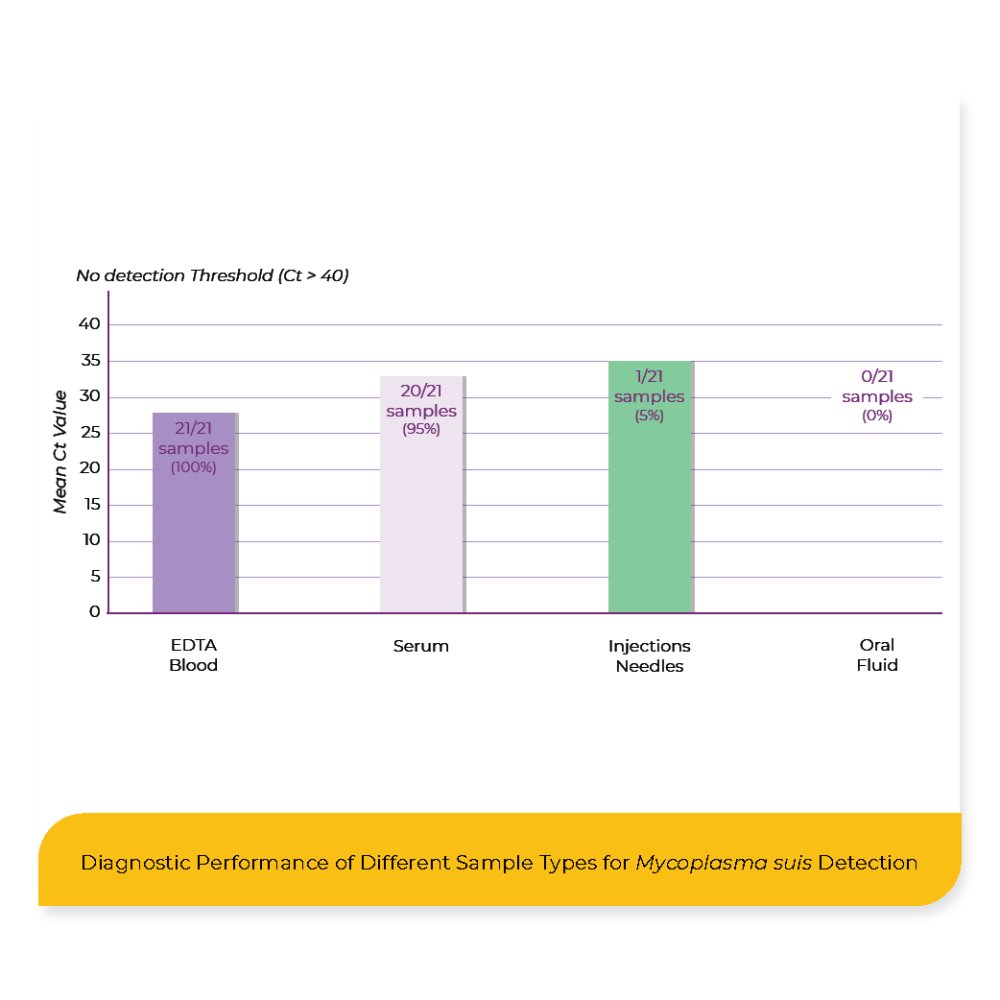
The results demonstrated that all 21 EDTA blood samples tested positive for M. suis, yielding the lowest average Ct value of 27.8. Serum samples yielded 95 % positive results in 20 out of 21 cases; however, the mean Ct value was higher at 32.3 compared to the EDTA blood samples. All oral fluid samples were negative, with no detectable M. suis DNA in any case. Notably, one of the 21 needle samples tested positive, with a Ct value of 35.1, indicating the presence of small amounts of pathogen DNA.
Discussion and Conclusion
The findings confirm EDTA blood as the most reliable and sensitive material for the molecular detection of M. suis, solidifying its status as the diagnostic gold standard. The consistently low Ct values and the 100 % detection rate underline its suitability for identifying M. suis infections.
Interestingly, serum samples also demonstrated a very high detection rate, with 20 out of 21 samples testing positive. Given that M. suis is a hemotrophic organism that adheres to erythrocytes, this result was unexpected, as serum is a cell-free matrix and is generally not recommended for M. suis diagnostics. The presence of detectable DNA in serum may reflect ongoing hemolysis or other mechanisms that warrant further investigation. Nonetheless, this finding opens up the possibility of using serum as a flexible diagnostic alternative in situations where EDTA blood is unavailable.
In contrast, the complete absence of M. suis DNA in oral fluid samples suggests that this non-invasive matrix is unsuitable for reliable detection.
The single positive finding from a used needle highlights the possibility of mechanical transmission and emphasizes the importance of hygienic practices in routine handling and injection procedures.
1Hoelzle LE, Felder KM, Hoelzle K: Porcine eperythrozoonosis: from Eperythrozoon suis to Mycoplasma suis. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2011, 39: 215-220
Let’s talk solutions and connect with our corresponding author: jennifer.reinmold@san-group.com
Credits:
J. Reinmold1, I. Spiekermeier1, S. Nowaczyk2, P. Cybulski3
1 SAN Group Biotech Germany GmbH, Hoeltinghausen, Germany
2 Uslugi Weterynaryjne lek.wet. Szymon Nowaczyk, Mieszków, Poland
3 Goodvalley Agro S.A., Przechlewo, Poland
SAN Group Biotech Germany GmbH
Mühlenstraße 13 I 49685 Höltinghausen GERMANY I +49 4473 9438 0 I office-de@san-group.com
Contact:
Contact us using the following form.

