Taking Mycotoxin Binder Validation in Pigs Beyond the Lab
Visit Official Website: Health and Nutritional Solutions for Pigs and Piglets
Taking Mycotoxin Binder Validation in Pigs Beyond the Lab
Mycotoxins, metabolites produced by fungi such as Fusarium, Aspergillus, and Penicillium, are a constant threat to pig health and productivity. In 2025, Kemin Customer Laboratory Service reported that 78% of complete feed samples contained at least one mycotoxin. Common contaminants include Zearalenone (ZEA), Ochratoxin A (OTA), trichothecenes (Deoxynivalenol, T-2, HT-2 toxins), Aflatoxins, and other mycotoxins. These toxins can cause a spectrum of effects in pigs, ranging from immune suppression and reproductive disorders to reduced growth and increased mortality. Even low-level contamination can reduce herd performance, making effective mycotoxin control essential.
Zearalenone and Ochratoxin A in Pigs
In this article, we focus on ZEA and OTA, two mycotoxins that present distinct threats to pig health. ZEA is a non-steroidal estrogenic mycotoxin that mimics estradiol and has significant effects on the reproductive system. In gilts and sows, even relatively low doses can trigger hyperestrogenism, anestrus, early embryonic loss, and reduced litter size. Conversely, OTA is a potent nephrotoxin that inhibits protein synthesis and induces oxidative stress. In pigs, dietary exposure to OTA can lead to renal damage, fibrosis, decreased feed intake, and a reduction in performance.
Reducing Mycotoxin Bioavailability in vivo
The selection of mycotoxin management solutions is typically based on in vitro binding efficacy. However, under real farm conditions, the complexity of animal physiology and other environmental factors means that in vitro results do not always predict actual efficacy. To address this, a toxicokinetic study was conducted using 12 piglets with an average body weight of 19.5 kg. TOXFIN®, a carefully selected blend of adsorbents, and specific mycotoxins were administered via intragastric tube at the following doses: 0.05 mg OTA/kg BW, 3 mg ZEN/kg BW, and 150 mg/kg BW of TOXFIN. The control group received only the mycotoxins, while the treatment group received both TOXFIN and the mycotoxins. Efficacy was assessed by quantifying mycotoxins and their metabolites in blood plasma at defined intervals to determine the impact on oral bioavailability, which represents the fraction of mycotoxins detected in the plasma of treated piglets compared to controls.
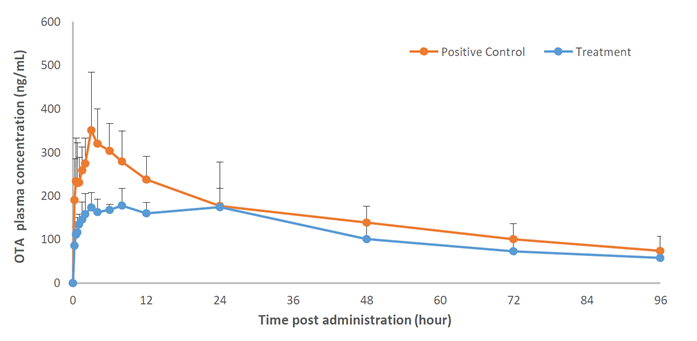
Figure 1: Plasma Ochratoxin A Levels (0.05 mg/kg BW) in Piglets: Control vs. TOXFIN
Figure 1 shows the plasma concentrations of OTA in both groups.
As showed for OTA in Figure 1, both ZEA and OTA are rapidly absorbed, reaching peak levels shortly after administration. TOXFIN effectively reduced the bioavailability of ZEA and OTA by 73.4% and 72.6%, respectively. This represents a significant reduction in exposure for pigs, as a clear correlation between feed contamination, blood levels, and organ impact has been demonstrated in the literature. Lower plasma levels following administration of the mycotoxin binder mean less accumulation in organs and a reduced risk of clinical effects. These results highlight the potential of the mycotoxin binder under very high exposure conditions to confirm its efficacy in pigs. The levels tested were approximately 20 times higher than the daily exposure expected under the EU Commission advisory limits for OTA and ZEA.
Conclusion
An effective mycotoxin management strategy in pig production is critical. While regular monitoring of raw materials and feed is essential, selecting binders that demonstrate a significant reduction in bioavailability under in vivo conditions can make the difference for animals and profitability.
Contact:
Contact us using the following form.








